G
Use of Environmental Tracers to Determine the Interaction of Ground Water and Surface Water
Environmental tracers are naturally occurring dissolved constituents, isotopes, or physical properties of water that are used to track the movement of water through watersheds. Useful environmental tracers include (1) common dissolved constituents, such as major cations and anions; (2) stable isotopes of oxygen (18O) and hydrogen (2H) in water molecules; (3) radioactive isotopes such as tritium (3H) and radon (222Rn); and (4) water temperature. When used in simple hydrologic transport calculations, environmental tracers can be used to (1) determine source areas of water and dissolved chemicals in drainage basins, (2) calculate hydrologic and chemical fluxes between ground water and surface water, (3) calculate water ages that indicate the length of time water and dissolved chemicals have been present in the drainage basin (residence times), and (4) determine average rates of chemical reactions that take place during transport. Some examples are described below.
 |
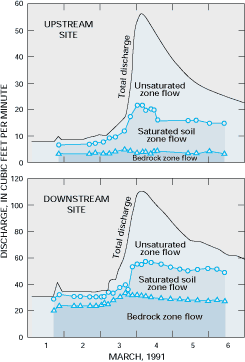
Major cations and anions have been used as tracers in studies of the hydrology of small watersheds to determine the sources of water to streamflow during storms (see Figure G-1). In addition, stable isotopes of oxygen and hydrogen, which are part of water molecules, are useful for determining the mixing of waters from different source areas because of such factors as (1) differences in the isotopic composition of precipitation among recharge areas, (2) changes in the isotopic composition of shallow subsurface water caused by evaporation, and (3) temporal variability in the isotopic composition of precipitation relative to ground water.
Radioactive isotopes are useful indicators of the time that water has spent in the ground-water system. For example, tritium (3H) is a well-known radioactive isotope of hydrogen that had peak concentrations in precipitation in the mid-1960s as a result of above-ground nuclear-bomb testing conducted at that time. Chlorofluorocarbons (CFCs), which are industrial chemicals that are present in ground water less than 50 years old, also can be used to calculate ground-water age in different parts of a drainage basin.
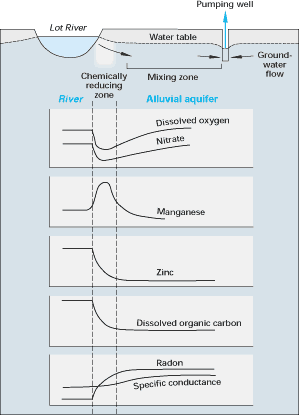
222Radon is a chemically inert, radioactive gas that has a half-life of only 3.83 days. It is produced naturally in ground water as a product of the radioactive decay of 226radium in uranium-bearing rocks and sediment. Several studies have documented that radon can be used to identify locations of significant ground-water input to a stream, such as from springs. Radon also has been used to determine stream-water movement to ground water. For example, radon was used in a study in France to determine stream-water loss to ground water as a result of ground-water withdrawals. (See Figure G-2.)
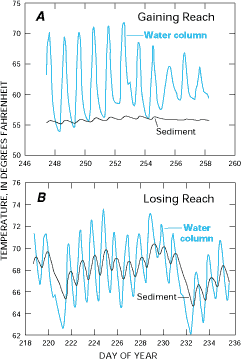
An example of using stream-water temperature and sediment temperature for mapping gaining and losing reaches of a stream is shown in Figure G-3. In gaining reaches of the stream, sediment temperature and stream-water temperature are markedly different. In losing reaches of the stream, the diurnal fluctuations of temperature in the stream are reflected more strongly in the sediment temperature.
Figure G-1. The relative contributions of different subsurface water sources to streamflow in a stream in Tennessee were determined by analyzing the relative concentrations of calcium and sulfate. Note that increases in bedrock zone (ground water) flow appear to contribute more to the stormflow response at the downstream site than to the stormflow response at the upstream site in this small watershed. (Modified from Mulholland, P.J., 1993, Hydrometric and stream chemistry evidence of three storm flowpaths in Walker Branch Watershed: Journal of Hydrology, v. 151, p. 291-316.) (Reprinted with permission from Elsevier Science-NL, Amsterdam, The Netherlands.)
Figure G-2. Sharp changes in chemical concentrations were detected over short distances as water from the Lot River in France moved into its contiguous alluvial aquifer in response to pumping from a well. Specific conductance of water was used as an environmental tracer to determine the extent of mixing of surface water with ground water, and radon was used to determine the inflow rate of stream water. Both pieces of information were then used to calculate the rate at which dissolved metals reacted to form solid phases during movement of stream water toward the pumping well. (Modified from Bourg, A.C.M., and Bertin, C., 1993, Biogeochemical processes during the infiltration of river water into an alluvial aquifer: Environmental Science and Technology, v. 27, p. 661-666.) (Reprinted with permission from the American Chemical Society.)
Figure G-3. Ground-water temperatures generally are more stable than surface-water temperatures. Therefore, gaining reaches of Juday Creek in Indiana are characterized by relatively stable sediment temperatures compared to stream-water temperatures (A). Conversely, losing reaches are characterized by more variable sediment temperatures caused by the temperature of the inflowing surface water (B). (Modified from Silliman, S.E., and Booth, D.F., 1993, Analysis of time series measurements of sediment temperature for identification of gaining versus losing portions of Juday Creek, Indiana: Journal of Hydrology, v. 146, p. 131-148.) (Reprinted with permission from Elsevier Science-NL, Amsterdam, The Netherlands.)