 |
 |
A Laboratory Manual for X-Ray Powder Diffraction
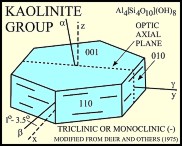
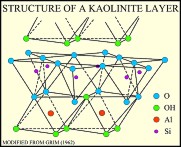 The kaolinite group includes the dioctahedral minerals kaolinite, dickite, nacrite, and halloysite, and the trioctahedral minerals antigorite, chamosite, chrysotile, and cronstedite. The primary structural unit of this group is a layer composed of one octahedral sheet condensed with one tetrahedral sheet. In the dioctahedral minerals the octahedral site are occupied by aluminum; in the trioctahedral minerals these sites are occupied by magnesium and iron. Kaolinite and halloysite are single-layer structures. Although dickite and nacrite have the same basic structure, the stacking sequence of layers is different in these minerals (Dixon, 1989; Moore and Reynolds, 1997). Kaolinite, dickite, and nacrite occur as plates; halloysite, which can have a single layer of water between its sheets, occurs in a tubular form.
The kaolinite group includes the dioctahedral minerals kaolinite, dickite, nacrite, and halloysite, and the trioctahedral minerals antigorite, chamosite, chrysotile, and cronstedite. The primary structural unit of this group is a layer composed of one octahedral sheet condensed with one tetrahedral sheet. In the dioctahedral minerals the octahedral site are occupied by aluminum; in the trioctahedral minerals these sites are occupied by magnesium and iron. Kaolinite and halloysite are single-layer structures. Although dickite and nacrite have the same basic structure, the stacking sequence of layers is different in these minerals (Dixon, 1989; Moore and Reynolds, 1997). Kaolinite, dickite, and nacrite occur as plates; halloysite, which can have a single layer of water between its sheets, occurs in a tubular form.
 All members of the kaolinite group form primarily during hydrothermal alteration or weathering of feldspars under acid conditions; but kaolinite and halloysite are probably the only members formed in soils (Deer and others, 1975; Swindale, 1975). Kaolin minerals are used during the manufacture of ceramics, paper, and paint.
All members of the kaolinite group form primarily during hydrothermal alteration or weathering of feldspars under acid conditions; but kaolinite and halloysite are probably the only members formed in soils (Deer and others, 1975; Swindale, 1975). Kaolin minerals are used during the manufacture of ceramics, paper, and paint.
In mono-mineralic samples the dioctahedral members of this group are readily identified because they become amorphous to X-rays after heating to 550 degrees C and their diffraction patterns disappear. Kaolinite, dickite, nacrite, and halloysite can be differentiated from chlorite by comparisons of the 3.58-angstrom kaolin peak with the 3.54-angstrom chlorite peak (Biscaye, 1965), and from chlorite and the trioctahedral members of this group by intercalation with potassium acetate (Wada, 1965). Heating alone will not distinguish the dioctahedral kaolinite group minerals from chlorite because the 002, 003, and 004 chlorite peaks are also weakened by this heat treatment (Moore and Reynolds, 1997). Intercalation complexes can also be used to differentiate individual dioctahedral kaolinite minerals. For example, dehydrated halloysite can be distinguished from kaolinite by intercalation with formamide (Churchman and others, 1984).
| X-ray powder diffraction patterns of oriented-aggregate mounts showing the effects of standard treatments on: | |