Data Series 301
U.S. GEOLOGICAL SURVEY
Data Series 301
Ground-water samples were collected using standard and modified USGS protocols (Koterba and others, 1995; U.S. Geological Survey, variously dated), and protocols described by Weiss, 1968; Shelton and others, 2001; Ball and McClesky, 2003a,b; and Wright and others, 2005. Prior to sampling, each well was pumped continuously in order to purge at least three casing-volumes of water from the well (Wilde and others, 1999). Wells were sampled using Teflon tubing with brass and stainless-steel fittings attached to a sampling point on the well discharge pipe as close to the well as possible. The sampling point was always located upstream of any well-head treatment system or water storage tank. If a chlorinating system was attached to the well, the chlorinator was shut off at least 24 hours prior to purging and sampling the well in order to clear all chlorine out of the system. For the fast and intermediate schedules, samples were collected at the well head using a foot-long length of Teflon tubing. For the slow schedule, the samples were collected inside an enclosed chamber located inside a mobile laboratory and connected to the well head by a 10- to 50- foot length of the Teflon tubing (Lane and others, 2003). All fittings and lengths of tubing were cleaned between samples (Wilde, 2004).
For the field measurements, ground water was pumped through a flow-through chamber fitted with a multi-probe meter that simultaneously measures the water-quality indicators—dissolved oxygen, temperature, pH, turbidity, and specific conductance. Field measurements were made in accordance with protocols in the USGS National Field Manual (Wilde and Radtke, 2005; Wilde, 2006; Lewis, 2006; Radtke and others, 2005; Wilde and others, 2006). All sensors on the multi-probe meter were calibrated daily. Measured temperature, dissolved oxygen, pH, and specific conductance values were recorded at 5-minute intervals for at least 30 minutes, and when these values remained stable for 20 minutes, samples for laboratory analyses were then collected. Field measurements and instrument calibrations were recorded by hand on field record sheets and electronically in PCFF-GAMA, a software package designed by the USGS with support from the GAMA program. Analytical service requests were also managed by PCFF-GAMA. Information from PCFF-GAMA was uploaded directly into NWIS at the end of every week of sample collection.
For analyses requiring filtered water, ground water was diverted through a 0.45-μm pore size vented capsule filter, a disk filter, or a baked glass-fiber filter depending on the protocol for the analysis (Wilde and others, 1999; Wilde and others, 2004). Prior to sample collection, polyethylene sample bottles were pre-rinsed two times using deionized water, and then once with sample water before sample collection. Samples requiring acidification were acidified to a pH of 2 or less with the appropriate acids using ampoules of certified, traceable concentrated acids obtained from the USGS National Water Quality Laboratory (NWQL).
Temperature-sensitive samples were stored on ice prior to, and during, daily shipping to the various laboratories. The non-temperature sensitive samples for tritium, noble gases, chromium speciation, and stable isotopes were shipped monthly, while volatile organic compounds, pesticides, compounds of special interest, dissolved organic carbon, radium isotopes, gross alpha and beta radioactivity, and radon-222 samples were shipped daily.
Detailed sampling protocols for individual analyses and groups of analytes are described in Koterba and others (2005) and the USGS National Field Manual (Wilde and others, 1999; Wilde and others, 2004) and in the references for analytical methods listed in table A1; only brief descriptions are given here. Volatile organic compounds (VOCs) and gasoline oxygenates and degradates, and 1,2,3-trichloropropane (1,2,3-TCP) samples were collected in 40-mL sample vials that were purged with three vial volumes of sample water before bottom filling to eliminate atmospheric contamination. Six normal (6 N) hydrochloric acid (HCl) was added as a preservative to the VOC samples, but not to the gasoline oxygenate and degradate samples, or the 1,2,3-TCP samples. The perchlorate sample was collected in a 125-mL polyethylene bottle. Tritium samples were collected by bottom filling two 1-L polyethylene bottles with unfiltered ground water, after first overfilling the bottle with three volumes of water. Stable isotopes of water were collected in 60-mL clear glass bottles filled with unfiltered water, sealed with a conical cap, and secured with electrical tape to prevent leakage and evaporation.
Pesticides and pesticide degradation products, wastewater-indicator constituents, pharmaceutical compounds, and N-nitrosodimethylamine (NDMA) samples were collected in 1-L baked amber bottles. Pesticide, wastewater-indicator, and pharmaceutical samples were filtered through a glass fiber during collection, whereas the NDMA samples were filtered at the Montgomery Watson-Harza Laboratory prior to analysis.
Ground-water samples for major and minor ions, trace elements, alkalinity, and total dissolved solids analyses required filling one 250-mL polyethylene bottle with raw ground water, and one 500-mL and one 250-mL polyethylene bottle with filtered ground water (Wilde and others, 2004). Filtration was done using a Whatman capsule filter. The 250-mL filtered sample was then preserved with 7.5 N nitric acid. Mercury samples were collected by filtering ground water into a 250-mL glass bottle and preserving with 6 N hydrochloric acid. Arsenic and iron speciation samples were filtered into a 250-mL polyethylene bottle that was covered with tape to prevent light exposure, and preserved with 6 N hydrochloric acid. The nutrient sample was filtered into a 125-mL brown polyethylene bottle. Radium isotopes and gross alpha and beta radiation samples were filtered into 1-L polyethylene bottles and acidified with nitric acid. Carbon isotope samples were filtered and bottom filled into two 500-mL glass bottles that were first overfilled with three bottle volumes of ground water. These samples had no headspace, and were sealed with a conical cap to avoid atmospheric contamination. Samples for alkalinity titrations were collected by filtering ground water into a 500-mL polyethylene bottle.
DOC, chromium, radon-222, dissolved gases, and microbial constituents were collected from the hose bib at the well head, regardless of the sampling schedule (fast, intermediate, or slow). DOC was collected after rinsing the sampling equipment with universal blank water (Wilde and others, 2004). Using a 50-mL syringe and 0.45-μm disk filter, the ground-water sample then was filtered into a 125-mL baked glass bottle and preserved with 4.5 N sulfuric acid. Chromium speciation samples were collected using a 10-mL syringe with an attached 0.45-μm disk filter. After the syringe was thoroughly rinsed and filled with ground water, 4 mL was forced through the disk filter; the next 2 mL of the ground water was slowly filtered into a small centrifuge vial for analysis of total chromium. Hexavalent chromium, Cr (VI), was then collected by attaching a small cation exchange column to the syringe filter, and after conditioning the column with 2 mL of sample water, 2 mL was collected in a second centrifuge vial. Both vials were preserved with 10 μL of 7.5 N nitric acid (Ball and McClesky, 2003a,b).
For the collection of radon-222, a stainless steel and Teflon valve assembly was attached to the sampling port at the well head (Wilde and others, 2004). The valve was partially closed to create back pressure, and a 10-mL sample was taken through a Teflon septum on the value assembly using a glass syringe affixed with a stainless steel needle. The sample was then injected into a 25-mL vial partially filled with scintillation mixture (mineral oil) and shaken. The vial was then placed in a cardboard tube in order to shield it from light during shipping.
Noble gases were collected in 3/8-in copper tubes using reinforced nylon tubing connected to the hose bib at the wellhead. Ground water was flushed through the tubing to dislodge bubbles before flow was restricted with a back pressure valve. Clamps on either side of the copper tube were then tightened, trapping a sample of ground water for analyses of noble gases (Weiss, 1968).
Samples for analysis of microbial constituents also were collected at the well head (Myers, 2004; Bushon, 2003). Prior to the collection of samples, the sampling port was sterilized using isopropyl alcohol, and ground water was run through the sampling port for at least three minutes to remove any traces of the sterilizing agent. Two sterilized 250-mL bottles were then filled with ground water for coliform analyses (total and Escherichia coliform determinations), and one sterilized 3-L carboy was filled for coliphage analyses (F specific and somatic coliphage determinations).
Ten laboratories performed chemical and microbial analyses for this study (see table A1), although most of the analyses were performed at the NWQL or by labs contracted by the NWQL. The NWQL maintains a rigorous quality assurance program (Maloney, 2005; Pirkey and Glodt, 1998). Laboratory quality control samples, including method blanks, continuing calibration verification standards, standard reference samples, reagent spikes, external certified reference materials, and external blind proficiency samples, are analyzed regularly. Method detection limits are continuously tested and laboratory reporting levels updated accordingly. NWQL maintains National Environmental Laboratory Accreditation Program (NELAP) and other certifications (http://nwql.usgs.gov/Public/Performance/publiclabcertcoverpage.html). In addition, the Branch of Quality Systems within the USGS Office of Water Quality maintains independent oversight of quality assurance at the NWQL and labs contracted by the NWQL. The Branch of Quality Systems also runs a national field quality assurance program that includes annual testing of all USGS field personnel for proficiency in making field water-quality measurements (http://nfqa.cr.usgs.gov/). Results for analyses made at the NWQL or by laboratories contracted by the NWQL are uploaded directly into NWIS by the NWQL. Laboratory quality-control data are also stored in NWIS.
Turbidity, alkalinity, and total coliforms and Escherichia coliform (E. coli) were measured in the mobile laboratory at the well site. Turbidity was measured in the field with a calibrated turbidity meter. Total coliforms and E. coli plates were prepared using sterilized equipment and reagents (Myers, 2004). Plates were counted under an ultraviolet light, following a 22-24 hour incubation time. Alkalinity and the concentrations of bicarbonate (HCO3-) and carbonate (CO32-) were measured on filtered samples by Gran’s titration method (Rounds, 2006).
Concentrations of HCO3- and CO32- were also calculated from the laboratory alkalinity and pH measurements. Calculations were made using the advanced speciation method (http://or.water.usgs.gov/alk/methods.html) with pK1 = 6.35, pK2 = 10.33, and pKW = 14.
The USGS NWQL uses the laboratory reporting level (LRL) as a threshold for reporting analytical results. The LRL is set to minimize the reporting of false negatives (not detecting a compound when it is actually present in a sample) to less than 1 percent (Childress and others, 1999). The LRL is set at two times the long-term method detection level (LT-MDL). The LT-MDL is derived from the standard deviation of at least 24 MDL determinations made over an extended period of time. LT-MDLs are continually monitored and updated. The method detection limit (MDL) is the minimum concentration of a substance that can be measured and reported with 99 percent confidence that the concentration is greater than zero (at MDL there is less than 1 percent chance of a false positive) (U.S. Environmental Protection Agency, 2002a). The USGS NWQL updates LRL values regularly and the values listed in this report were in effect during the period analyses were made for ground-water samples from the SOSA study (June and July, 2006).
Some compound concentrations in this study are reported using minimum reporting levels (MRLs) or method uncertainties. The MRL is the smallest measurable concentration of a constituent that may be reliably reported using a given analytical method (Timme, 1995). The method uncertainty generally indicates the precision of a particular analytical measurement; it gives a range of values wherein the true value will be found.
Detections between the LRL and the LT-MDL are reported as estimated concentrations (designated with an “E” before the values in the tables and text). For information-rich methods, detections below the LRL have high certainty of detection, but the precise concentration is uncertain. Information-rich methods are those that utilize gas chromatography or high-performance liquid chromatography (HPLC) with mass spectrometry detection (VOCs, gasoline oxygenates and degradates, pesticides, pharmaceuticals, and wastewater-indicators). Compounds are identified by presence of characteristic fragmentation patterns in their mass spectra in addition to being quantified by measurement of peak areas at their associated chromatographic retention times. E-values also may result from detections outside the range of calibration standards, for detections that did not meet all laboratory quality-control criteria, and for samples that were diluted prior to analysis (Childress and others, 1999).
Detections that may have resulted from sample contamination are reported with a “V” before the values in the tables. The potential for sample contamination was assessed using results from field, source, and laboratory blanks.
The reporting levels for radiochemical constituents (gross-alpha radioactivity, gross-beta radioactivity, radium-226, and radium-228) are based on a sample-specific minimum detectable concentration (SSMDC), a sample-specific critical value, and the combined standard uncertainty (CSU) (U.S. Environmental Protection Agency, 2004; Bennett and others, 2006). A result above the critical value represents a greater-than-95-percent certainty that the result is greater than zero (significantly different from the instrument’s background response to a blank sample), and a result above the SSMDC represents a greater-than-95-percent certainty that the result is greater than the critical value. Using these reporting level elements, three unique cases are possible when screening the raw analytical data. If the analytical result is less than the critical value (case 1), the analyte is considered not detected, and the concentration is reported as less than the SSMDC. If the analytical result is greater than the critical value, the ratio of the CSU to the analytical result is calculated as a percent (percent relative CSU). For those samples with percent relative CSU greater than 20 percent, concentrations are reported as estimated values (designated by an “E” preceding the value) (case 2). For those samples with percent relative CSU less than 20 percent, concentrations are reported unqualified (case 3).
Stable isotopic compositions of oxygen, hydrogen, and carbon are reported as relative isotope ratios in units of per mil using the standard delta notation (Coplen and others, 2002):
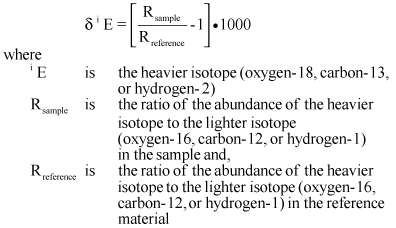
The reference material for oxygen and hydrogen is Vienna Standard Mean Ocean Water (VSMOW), which is assigned δ18O and δ2H values of 0 per mil (note than δ2H is also written as δD because the common name of the heavier isotope of hydrogen, hydrogen-2, is deuterium). The reference material for carbon is Vienna Peedee Belemnite (VPDB), which is assigned a δ13C value of 0 per mil. Positive values indicate enrichment of the heavier isotope and negative values indicate depletion of the heavier isotope, compared to the ratios observed in the standard reference material.
Twenty-seven constituents targeted in this study are measured by more than one analytical schedule or more than one laboratory (table A2). Results from certain analytical schedules are preferred over others because the methodology is more accurate or precise and generally yields greater sensitivity for a given compound.
The preferred methods for the nineteen constituents analyzed under multiple analytical schedules at the NWQL were selected based on the procedure recommended by the NWQL (http://wwwnwql.cr.usgs.gov/USGS/Preferred_method_selection_procedure.html). This procedure generally results in the preferred method being the one with the lower reporting limit. The nineteen constituents each appear on two of the following analytical schedules: VOCs (Schedule 2020), gasoline oxygenates and degradates (Schedule 4024), pesticides (Schedule 2003), pharmaceutical compounds (Schedule 2080), and wastewater-indicator compounds (Schedule 1433) (table A2). Only the values determined by the preferred method are reported. This report uses Schedule 2080 (table 3D) as the preferred method for caffeine because it has the lower detection limit and for data consistency; however, as of 2007, NWQL considered Schedule 1433 (table 3E) the preferred method because Schedule 2080 only became an NWQL method in 2005 and therefore has a relatively short history of quality-assurance data. All ground-water samples were analyzed for pharmaceuticals (Schedule 2080), but only six were analyzed for wastewater-indicator constituents (Schedule 1433).
The water-quality indicators—pH, specific conductance, and alkalinity—were measured in the field and at the NWQL. The field measurements are the preferred method for all three constituents; however, laboratory alkalinity results were used in this report because fewer samples had field alkalinity measurements.
The field and laboratory data were compared using the Wilcoxon signed-rank test, a non-parametric statistical test that is analogous to the parametric statistical test, the paired t-test (Helsel and Hirsch, 2002). A non-parametric test was used because the data are not normally distributed. The Wilcoxon signed-rank test evaluates the null hypothesis that the median of the paired differences between the two data sets is zero. Results are reported as the probability, P, of obtaining the observed distribution of data, or one even less likely, when the null hypothesis is true. Therefore, a P value of 0.01 indicates 99 percent confidence that the two data sets are different.
Specific conductance was measured in both the field and the laboratory for twenty-two samples, and there was no difference between the two data sets (P = 0.19). Both laboratory and field pH measurements were made for ten samples, and the two data sets were systematically different (P = 0.006). Field pH values were lower by a median of 0.3 pH units. The increase in pH between field and laboratory measurement may be explained by equilibration of the sample with the atmosphere after collection. The partial pressure of CO2 in ground water is often greater than the atmospheric partial pressure (Appelo and Postma, 2005), thus CO2 degasses from the ground water when it is brought in contact with the atmosphere. CO2 loss results in increased pH. Field and laboratory alkalinities were measured for seven samples, and the two data sets were not significantly different (P = 0.035). Field alkalinity values were lower by a median of 5 mg/L as CaCO3, but the differences between the field and laboratory values were less than 4 percent for all but one sample, which was within the error of the alkalinity measurement (Rounds, 2006).
For arsenic, chromium, and iron concentrations, the standard methods used by the NWQL are preferred over the research methods used by the USGS Trace Metal Laboratory. The concentrations measured by the Trace Metal Laboratory are used only to calculated ratios of redox species for each element, ![]() for arsenic,
for arsenic, ![]() for chromium, and
for chromium, and ![]() for iron. For example:
for iron. For example:

Blank samples (blanks) were collected using water (Nitrogen-Purged Universal blank water) certified by the NWQL to contain less than the LRL or MRL of the analytes investigated in the study. Two types of blanks were collected: source-solution and field blanks. Source-solution blanks were collected to verify that the blank water used for the field blanks was free of analytes. Field blanks were collected at 12 percent of the wells sampled and source solution blanks at 8 percent of the wells sampled to determine if equipment or procedures used in the field or laboratory introduced contamination. Field blanks were analyzed for VOCs; gasoline oxygenates and degradates; pesticides; pharmaceuticals; perchlorate; NDMA; 1,2,3-TCP; nutrients; dissolved organic carbon; major and minor ions; trace elements; iron, arsenic, and chromium speciation; and radioactive constituents (table A3). Universal blank water is not available for tritium or noble gases, thus field blanks were not collected for these constituents.
Source-solution blanks were collected at the sampling site by pouring blank water directly into sample containers that were preserved, stored, shipped, and analyzed in the same manner as the ground-water samples. For field blanks, blank water was either pumped or poured through the sampling equipment (fittings and tubing) used to collect ground water, then processed and transported using the same protocols for the ground-water samples. The equipment used to collect samples from wells on the slow schedule was different than the equipment used to collect samples from wells on the fast and intermediate schedules; therefore, detections in field blanks collected at slow wells were compared with detections in ground-water samples from slow wells, and detections in field blanks collected at fast and intermediate wells were compared with detections in ground-water samples from fast and intermediate wells.
If a constituent was detected in a field blank, the associated source-solution blank results were examined for similar constituent detections. If the field blank and the source-solution blank contained the constituent, then the source solution water was interpreted as the origin of the contamination in the blanks, and the field blank detections using the same blank water were disregarded. If the sample collected just prior to the contaminated field blank had high concentrations of the constituents in question, carry-over was considered to be the cause of the contamination.
If the presence of a constituent in a field blank could not be accounted for by contamination of the source-solution, carry-over, or a specific problem recorded in the field notes, then that field blank detection was used to censor detections in all ground-water samples collected with the same equipment. The censoring level was defined as the concentration of the constituent in the field blank plus one-half the LRL for that constituent. Detections in ground-water samples below the censoring level were censored. Censored values are indicated by a ‘V’ preceding the value in the tables, and are excluded from the summary statistics.
Sequential replicate samples were collected to assess variability that may result from the processing and analyses of inorganic and organic constituents. Relative standard deviation (RSD) of the measured values was used in determining the variability between replicate pairs for each compound (tables A4A–D). The RSD is defined as 100 times the standard deviation divided by the mean concentration for each replicate pair of samples. If one value in a sample pair was reported as a non-detection and the other value was reported as an estimate below the LRL or MRL, the RSD was set to zero because the values are analytically identical. If one value in a sample pair was reported as a non-detection and the other value was greater than the LRL or MRL, then the non-detection value was set equal to one-quarter of the LRL and the RSD was calculated (Hamlin and others, 2002). Values of RSD less than 20 percent are considered acceptable in this study. An RSD value of 20 percent corresponds to a relative percent difference (RPD) value of 29 percent. High RSD values for a compound may indicate analytical uncertainty at low concentrations, particularly for concentrations within an order of magnitude of LT-MDL or MDL. Sequential replicate samples were collected at 18 percent of the wells sampled.
Addition of a known concentration of a constituent (‘spike’) to a replicate environmental sample enables the analyzing laboratory to determine the effect of the matrix, in this case ground water, on the analytical technique used to measure the constituent. The known compounds added in matrix spikes are the same as those being analyzed in the method. This enables an analysis of matrix interferences on a compound by compound basis. Matrix spikes were added at the laboratory performing the analysis. Compounds with low recoveries are of potential concern if environmental concentrations are close to the MCLs; a concentration below an MCL could be falsely indicated. Conversely, compounds with high recoveries are of potential concern if the environmental concentrations exceed MCLs: a high recovery could falsely indicate a concentration above the MCL.
Acceptable ranges for matrix-spike recoveries are based on the acceptable ranges established for laboratory “set” spike recoveries. Laboratory set spikes are aliquots of laboratory blank water to which the same spike solution used for the matrix spikes has been added. One set spike is analyzed with each set of samples. Acceptable ranges for set spike recoveries are 70 to 130 percent for NWQL schedules 2020, 4024, and 1433 (Connor and others, 1998; Rose and Sandstrom, 2003; Zaugg and others, 2002), 60 to 120 percent for NWQL schedule 2003 (Sandstrom and others, 2001), and 60 to 130 percent for schedule 2080 (Kolpin and others, 2002). Based on these ranges, we defined 70 to 130 percent as the acceptable range for matrix-spike recoveries for organic compounds in this study.
Matrix spikes were performed for VOCs, gasoline oxygenates and degradates, pesticides, pharmaceuticals, wastewater-indicators, NDMA, and 1,2,3-TCP because the analytical methods for these constituents are chromatographic methods that may be susceptible to matrix interferences. Replicate samples for matrix-spike additions were collected at 12 percent of the wells sampled, although not all analyte classes were tested at every well (tables A5A–E).
Surrogate compounds are added to environmental samples in the laboratory prior to analysis in order to evaluate the recovery of similar constituents. Surrogate compounds were added to all ground-water and quality-control samples that were analyzed for VOCs, gasoline oxygenates and degradates, pesticides, pharmaceuticals, wastewater-indicators, NDMA, and 1,2,3-TCP (table A6). Most of the surrogate compounds are deuterated analogs of compounds being analyzed. For example, the surrogate toluene-d8 used for the VOC analytical method has the same chemical structure as toluene, except that the eight hydrogen-1 atoms on the molecule have been replaced by deuterium (hydrogen-2). Toluene-d8 and toluene behave very similarly in the analytical procedure, but the small mass difference between the two results in slightly different chromatographic retention times, thus the use of a toluene-d8 surrogate does not interfere with the analysis of toluene (Grob, 1995). Only 0.015 percent of hydrogen atoms are deuterium (Firestone and others, 1996), thus deuterated compounds like toluene-d8 do not occur naturally and are not found in environmental samples. Surrogates are used to identify general problems that may arise during sample analysis that could affect the analysis results for all compounds in that sample. Potential problems include matrix interferences (such as high levels of dissolved organic carbon) that produce a positive bias, or incomplete laboratory recovery (possibly due to improper maintenance and calibration of analytical equipment) that produces a negative bias. A 70 to 130 percent recovery of surrogates is generally considered acceptable; values outside this range indicate possible problems with the processing and analysis of samples (Connor and others, 1998; Sandstrom and others, 2001).
Field blanks were collected at approximately 12 percent of the sites sampled in SOSA. Table A3 presents a summary of detections in field blanks. The only VOC observed in field blanks was toluene. Toluene was detected in one of the six field blanks at a concentration of E0.02 µg/L. The field blank with the detection was collected using the “fast” and “intermediate” schedule equipment. However, low levels of toluene have been detected in source-solution and field blanks collected using both the “slow” and “intermediate” and “fast” equipment sets in many of the earlier study units (Wright and others, 2005; Kulongoski and others, 2006; Bennett and others, 2006; Dawson and others, 2007; Kulongoski and Belitz, 2007). Thus, the detection in the field blank was used to censor data collected using both equipment sets. Toluene was detected at a concentration of E0.02 µg/L in two ground-water samples; both of these detections were censored (tables 5 and A3).
Field blanks were collected at three of the twenty-two sites sampled for analysis of trace elements. Chromium was detected in all three field blanks at concentrations of E0.02, E0.02, and E0.03 µg/L. The field blank with a chromium concentration of E0.03 µg/L was accompanied by a source-solution blank that also had a concentration of E0.03 µg/L. The lowest concentration detected in ground-water samples was 0.04 µg/L; thus, no chromium data were censored on the basis of detections in the field blanks. Zinc was detected in two of three field blanks at concentrations of E0.5 µg/L and 1.40 µg/L. Three detections of zinc in ground-water samples with concentrations less than 1.7 µg/L (1.4 µg/L plus one-half the LRL of 0.6 µg/L) were therefore censored (tables 11 and A3). Barium and lead were each detected in one field blank, but at concentrations lower than found in the environmental samples, thus no data were censored. Copper and nickel were detected in one field blank at concentrations of 0.94 µg/L and 0.29 µg/L, respectively. The field blank containing copper and nickel and was collected using the “intermediate” schedule equipment. Six detections of copper in ground-water samples collected using the “intermediate” schedule equipment with concentrations less than 1.1 µg/L (0.94 µg/L plus one-half the LRL of 0.4 µg/L), and three detections of nickel with concentrations less than 0.32 µg/L (0.29 µg/L plus one-half the LRL of 0.03 µg/L) were therefore censored (tables 11 and A3). This resulted in censoring of three detections of copper and one detection of nickel in ground-water samples (tables 11 and A3).
Field blanks were collected at five of the fifty sites sampled for chromium species analysis at the USGS Trace Metal Laboratory (TML) and two of the twenty-two sites sampled for arsenic and iron species at the TML. One source-solution blank was collected for chromium, iron, and arsenic species analysis. None of the field or source-solution blanks contained iron or chromium. One field blank contained 2.3 µg/L of arsenic. The source-solution blank contained 4.3 µg/L of arsenic, but arsenic was not detected in the source solution blank and field blank collected at the same time for analysis by the NWQL. Five detections of arsenic in ground-water samples analyzed by the TML at concentrations less than 2.55 µg/L (2.3 mg/L plus one-half the MDL of 0.5 mg/L) were censored (tables 12 and A3).
DOC was detected in one field blank at a concentration of E0.2 mg/L, and low concentrations of DOC were detected in field blanks collected in previous GAMA study units (Kulongoski and Belitz, 2007; Bennett and others, 2006). Thus, the data for all ground-water samples with concentrations of E0.2 mg/L and E0.3 mg/L were censored (tables 9 and A3).
One field blank was collected for analysis of radioactive constituents. Radium-226 was detected at an activity of E0.02 pCi/L in the field blank. The one detection of radium-226 at an activity less than the activity measured in the blank was censored (table 15 and A3). No other radioactive constituents were detected in the field blank.
No compounds were detected in field blanks for the following analyte groups: pesticides and pesticide degradates (five field blanks), pharmaceutical compounds (six field blanks), wastewater-indicator compounds (one field blank), perchlorate (three field blanks), NDMA and 1,2,3-TCP (two field blanks), major and minor ions (three field blanks), and nutrients (three field blanks).
Tables A4A–D summarize the results of replicate analyses for constituents detected in ground-water samples collected in the SOSA study. Nearly 300 replicate analyses were made for constituents detected in at least one ground-water sample. Replicate analyses that were non-detections are not reported in tables A4A–D. Concentrations or activities in the environmental and replicate samples are reported for all replicate analyses with RSD values greater than zero. Most replicate analyses had RSD values less than 5 percent and only twelve had RSD values greater than the acceptable limit of 20 percent. Constituents with replicate analyses with RSD values greater than 20 percent include acetaminophen (tableA4A), perchlorate (table A4A), DOC (table A4B), aluminum (table A4C), zinc (table A4C), tritium (table A4D), and iron, arsenic, and chromium analyzed by the USGS Trace Metal Laboratory (table A4C). However, with the exception of one zinc replicate analysis, the magnitudes of the concentrations of the replicate sample pairs with RSD values greater than 20 percent were all within a factor of five of the LRLs for the respective analytes. At these low concentrations, small deviations in measured values result in large RSDs. The tritium replicate analyses were within laboratory analytical uncertainty of one another. Only four replicate analyses included one detection and one non-detection (aluminum, acetaminophen, and iron and arsenic analyzed at the Trace Metal Laboratory) and the detected concentrations were less than twice the LRLs. No data were censored as a result of variability in replicate analyses.
Tables A5A–E present a summary of matrix-spike recoveries for the SOSA study. Addition of a spike or known concentration of a constituent to an environmental sample enables the analyzing laboratory to determine the effect of the matrix, in this case ground water, on the analytical technique used to measure the constituent. Six environmental samples were spiked with VOCs to calculate matrix-spike recoveries (table A5A). Sixty-eight of the eighty-eight VOC spike compounds had recoveries within the acceptable range of 70 and 130 percent. Fifteen VOC spike compounds had at least one matrix-spike recovery greater than 130 percent; however, only two of these compounds were detected in ground-water samples. All six matrix-spike recoveries for carbon tetrachloride (tetrachloromethane) were greater than 130 percent (median 188 percent), suggesting that measured concentrations in environmental samples may be disproportionately high. An “E” code was given to the one detection of carbon tetrachloride to indicate that the magnitude of the concentration is uncertain (table 5). Of the six matrix spikes for trichlorofluoromethane (CFC-11) only two had recoveries greater than 130 percent and neither of the two environmental samples with trichlorofluoromethane detections was analyzed in the same batch as the matrix spike with high recoveries. Thus, the trichlorofluormethane detections were not flagged. Dichlorodifluoromethane, dichloromethane, 1,2,3,4-tetramethybenzene, 1,2,3,5-tetramethylbenzene, and 1,2,3-trichlorobenzene had median matrix-spike recoveries below 70 percent, but these compounds were not detected in ground-water samples (table 12). [NOTE – low recoveries may indicate that the compound might not have been detected in some samples if it was present at very low concentrations].
Five ground-water samples were spiked with pesticide and pesticide degradate compounds in order to calculate matrix-spike recoveries. Twenty-eight of the sixty-three spike compounds had recoveries within the acceptable range of 70 and 130 percent (table A5B). All five of the compounds detected in ground-water samples had spike recoveries within the acceptable range. Three spike compounds had at least one recovery greater than 130 percent. Thirty-three spike compounds had at least one recovery below 70 percent, and the median recovery was below 70 percent for fifteen compounds. [NOTE – low recoveries may indicate that the compound might not have been detected in some samples if it was present at very low concentrations].
Four ground-water samples were spiked with pharmaceutical compounds. Eight of the fourteen pharmaceutical compounds had recoveries within the acceptable range of 70 to 130 percent, and six compounds had recoveries less than 70 percent for at least one of the four tests (table A5C). Two compounds detected in ground-water samples, diphenhydramine and sulfamethoxazole, had low recoveries in all four spiked samples. [NOTE – low recoveries may indicate that the compound might not have been detected in some samples if it was present at very low concentrations].
One ground-water sample was spiked with wastewater-indicator compounds. Sixteen of the sixty-two compounds had recoveries less than 70 percent and one had recovery greater than 130 percent (table A5D). One of the compounds detected in ground-water samples, tetrachloroethene (PCE), had a recovery of only 15 percent, but the wastewater-indicator analytical method (NWQL schedule 1433) is not the preferred analytical method for tetrachloroethene (table A2).
Three ground-water samples were spiked with NDMA and 1,2,3-TCP. All spike recoveries were within the acceptable range of 70 to 130 percent (table A5E).
Surrogate compounds were added to environmental samples in the laboratory and analyzed to evaluate the recovery of similar constituents. Table A6 lists the surrogate, analytical schedule on which it was applied, the number of analyses for blank and non-blank samples, the number of surrogate recoveries below 70 percent, and the number of surrogate recoveries above 130 percent for the blank and non-blank samples. Blank and non-blank samples were considered separately to assess whether the matrices present in non-blank samples affect surrogate recoveries. No systematic differences between surrogate recoveries in blank and non-blank samples were observed. All surrogate recoveries in analyses of pharmaceutical compounds were in the acceptable range of 70 to 130 percent recovery, as were 90 percent of the surrogate recoveries for VOC and gasoline oxygenate and degradate analyses, and 93 percent of the surrogate recoveries for pesticide, NDMA, and 1,2,3-TCP analyses. Two-thirds of the surrogate recoveries for wastewater-indicator constituent analyses were in the acceptable range.
Three environmental samples with detections of VOCs had recoveries of the surrogate 1,2-dichloroethane-d4 greater than 130 percent. The three VOCs detected in these three samples all elute near 1,2-dichloroethane-d4 in the chromatographic sequence. A high recovery for a surrogate suggests that the measured concentrations of analytes eluting near the surrogate may be biased to higher concentrations. All three detections already had ‘E’ codes, thus no additional flagging was needed.
The protocols for analysis of pharmaceutical compounds (NWQL schedule 2080) has been used for routine sample analysis since October 2005, but due to the newness of the method compared with the other methods of analysis used in this study, an extra level of quality-control assessment was applied to the pharmaceutical data. In addition to the results for field blanks, matrix-spike recoveries, surrogates recoveries, and replicate variability, results for internal laboratory quality-control samples that were run with SOSA ground-water samples were compiled and examined. The fifty ground-water samples were analyzed in eleven different laboratory sets. Each set also included a set blank and a set spike. Purified water (de-ionized, carbon-filtered, and ultraviolet-radiation sterilized) was used for the set blanks and the matrix water for the set spikes (Kolpin and others, 2002).
Ten of the eleven set blanks had no detections of any pharmaceutical compounds. Diphenhydramine and carbamazapine were detected in one set blank at concentrations of E0.0005 µg/L and E0.0006 µg/L, respectively (table A7A). Detections in a set blank may indicate contamination of laboratory equipment that may affect other samples analyzed in the same set. Diphenhydramime and carbamazapine were each detected once in ground-water samples analyzed in the same set as the set blank containing those constituents. The concentrations in the ground-water samples were more than ten times the concentrations in the set blanks, thus the detections in the ground-water samples were not censored on this basis. However, examination of set blanks for laboratory sets containing GAMA ground-water samples collected between October 2005 and March 2007 showed that diphenhydramine was consistently detected in more than 20 percent of the set blanks. Due to this unacceptably high rate of low-level contamination, all detections of diphenhydramine were censored, even if the concentration in the ground-water sample was more than ten times greater than the concentration in the set blanks.
Recoveries of spike constituents in the set spike samples (table A7B) were similar to those in the ground-water matrix-spike samples (table A5C). Six of the fourteen pharmaceutical compounds had recoveries within the acceptable range of 70 to 130 percent in all eleven set spikes. Of the remaining eight compounds, four had median recoveries within the acceptable range. Median recoveries for acetaminophen, diltiazem, diphenhydramine, and warfarin were below the acceptable range. [NOTE – low recoveries may indicate that the compound might not have been detected in some samples if it was present at very low concentrations].