Indoor radon measurements were made using activated charcoal detectors that were sent to a laboratory for analysis. The detector placement, data recording, and detector recovery followed the USEPA protocols for activated charcoal detectors. The detectors were placed in the rooms to be tested during the evening hours of Friday and were recovered during the evening hours of the following Sunday. The dates and times of placement and recovery were recorded on appropriate data sheets along with room and school information. After collection, the detectors were sealed, and as soon as practical, the detectors were mailed to the laboratory for analysis. For approximately every ten rooms tested, a duplicate measurement was made, and for every twenty rooms, a blank detector that had not been exposed was submitted for analysis. In most instances, the detectors were placed on tables or desks near the center of the room. At schools found to have high indoor radon levels, detectors were placed inside cabinets or near possible radon entry points in selected rooms. These specially placed detectors were intended to whether higher radon concentrations could be measured near suspected entry points (e.g. see the results for Montemalaga Elementary).
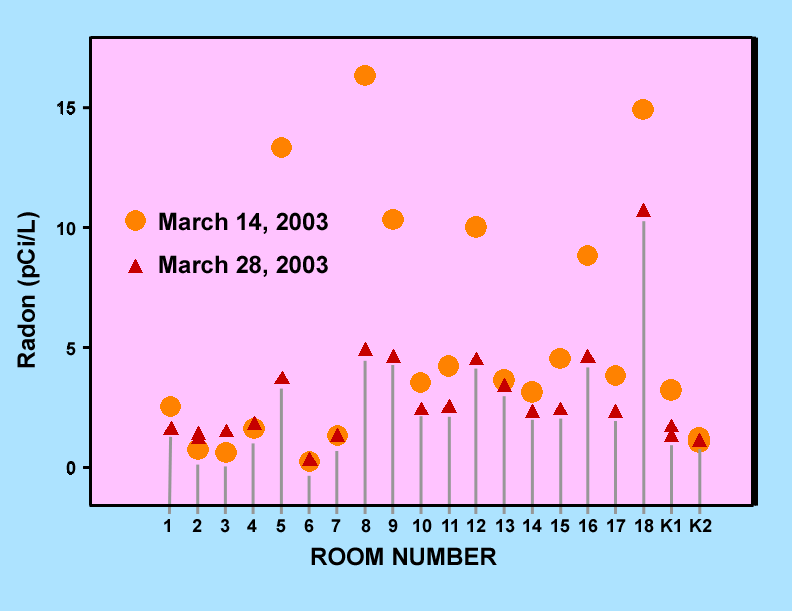
Figure 1 is a graph showing indoor radon levels at Montemalaga Elementary measured over two different time periods beginning on the dates indicated on the graph. Figure 2 shows similar data for Palos Verdes High School rooms of the 200 Science Wing. Both of these graphs have variations of more than a factor of two for some of the classrooms. These variations are probably typical of short-term screening measurements and reflect the effects of changes in a variety of environmental conditions such as atmospheric pressure, rainfall, and both inside and outside air temperatures. The accuracy of the actual measurements is estimated to be better than 0.5 pCi/L based upon the results of the blank measurements.

Figure 1. Indoor radon levels measured in classrooms at Montemalaga Elementary during two different time periods beginning on the dates indicated on the graph.
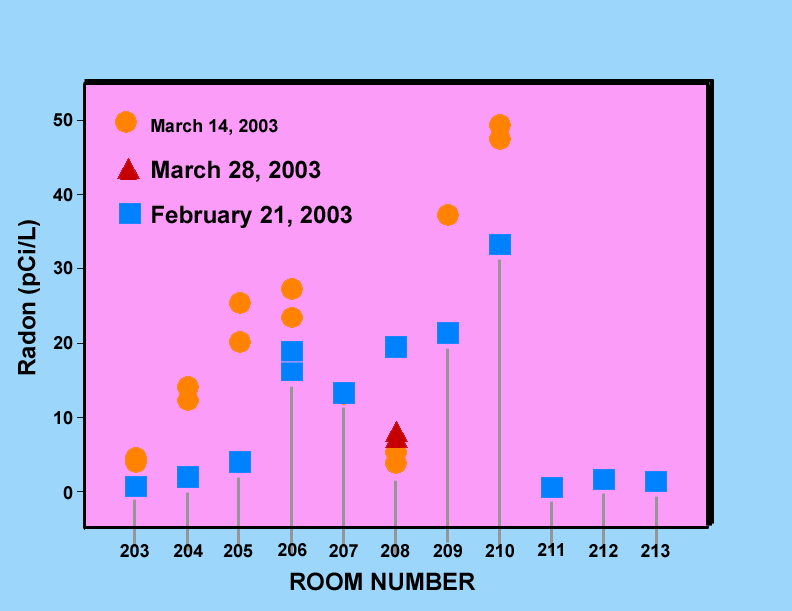
Figure 2. Indoor radon levels measured in classrooms of the 200 Science Wing at Palos Verdes High School during three different time periods beginning on the dates indicated on the graph.
Field gamma-ray spectrometer measurements were made using two portable gamma-ray spectrometers. One of the spectrometers had a detector with a volume of approximately 1.4 L of thallium activated sodium iodide (NaI(Tl)) and the other had a detector with a volume of about 3.3 L. Both spectrometers collected data in 512 channels and the measurement time used was five minutes. For most of the measurements, the detectors were suspended about 1 m above the ground using a wooden tripod. Suspending the detectors results in averaging the measurement over a circle about 10 m in diameter. In some cases, the area of open ground was less than 10 m in diameter and the detectors were placed directly on the ground to ensure a measurement representative of the soil. In either case, the depth of measurement is about 30 cm. Click here to view a picture of the field spectrometer and tripod.
The concentrations of potassium, uranium, and thorium were calculated using the counts accumulated in energy windows corresponding to the 1.46-MeV gamma ray of 40K, the 1.76-MeV gamma-ray of 214Bi, and the 2.64-MeV gamma ray of 208Tl, respectively. Figure 3 shows a typical gamma-ray spectrum with the energy windows used for potassium, uranium, and thorium measurements.
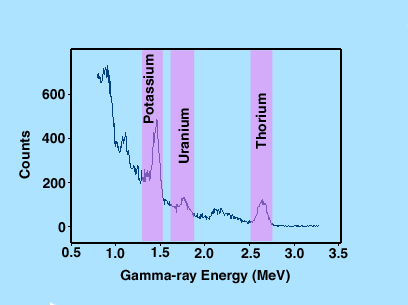
Figure 3. Typical gamma-ray spectrum from a field gamma-ray spectrometer with energy windows used for potassium, uranium, and thorium measurements.
Because the decay series of uranium and thorium are complex with many different chemical elements, these series may not be in radioactive equilibrium. If the decay series are not in radioactive equilibrium, measurements of the activity of a daughter product will not reflect the activity of the parent nuclei. Because we are measuring gamma rays from daughter products of uranium (214Bi) and thorium (208Tl) and assuming radioactive equilibrium, we acknowledge the possibility of disequilibrium by denoting the concentrations of uranium and thorium as equivalent uranium (eU) and equivalent thorium (eTh).
Both of the field gamma-ray spectrometers were calibrated by making measurements on concrete calibration pads with known concentrations of potassium, uranium, and thorium. These calibration pads are located at the U.S. Geological Survey headquarters in Reston, Virginia. We estimate the absolute accuracy of the field measurements to be better than 25 percent with a relative accuracy better than 15 percent.
Laboratory measurements of soil samples were done using two laboratory gamma-ray spectrometers located in a U.S. Geological Survey laboratory in Reston, Virginia. These spectrometers consist of NaI(Tl) detectors housed inside a structure of lead bricks that attenuate gamma rays from the surrounding materials of the laboratory. An on-line report by Snyder and Duval (2003) is available for those interested in details about the laboratory spectrometers (http://pubs.usgs.gov/of/2003/of03-029/).
Calibration of the laboratory spectrometers is accomplished via measurements of standard samples with known concentrations of potassium, uranium, and thorium. The soil samples collected at sites on the Palos Verdes Peninsula were sieved to 2 mm and placed in sample containers without any other manipulation of the samples. After the samples were put into the sample containers and sealed, they were immediately placed in the gamma-ray spectrometers and measured for a period of 26,000 seconds (7.22 hours). They were then set aside and reanalyzed after a period of about 21 days in order to ensure radioactive equilibrium of the radon (222Rn has a half-life of 3.82 days). The purpose of these two measurements was to obtain an estimate of the fraction of radon produced by the samples that escaped from the samples. We estimate the absolute accuracy of the laboratory measurements to be better than 10 percent.
Although the laboratory measurements are more accurate than the field measurements, the samples are not necessarily representative of the volume of material measured by the field spectrometers. The laboratory samples are generally 500-600 grams of soil whereas the field spectrometer measures a volume of approximately 100 m3 of soil (about 1,400,000 grams of soil, assuming 1.4 g/cm3 in situ soil density). Sample collection was done by mixing soil from four to six locations around the site of the field gamma-ray measurement and then extracting about 3 kg of sample. All samples were placed in plastic bags that were sealed and placed inside a second plastic bag that was also sealed.
Figures 4, 5, and 6 show graphs of field gamma-ray measurements of the potassium, uranium, and thorium concentrations versus laboratory measurements of the corresponding samples. Each graph includes the data points plotted as filled circles, a regression line plotted as a solid line, and 95 percent confidence limits plotted as dashed lines. In the case of potassium, three data points were rejected because the samples were judged to not be representative samples for determination of potassium. Similarly for thorium, two data points were rejected. These judgements were based upon the fact that the rejected data points biased the regression lines and were not consistent with the remaining points. Analysis of variance results for all of the regression lines indicate that the lines are statistically significant at a confidence level greater than 99 percent.
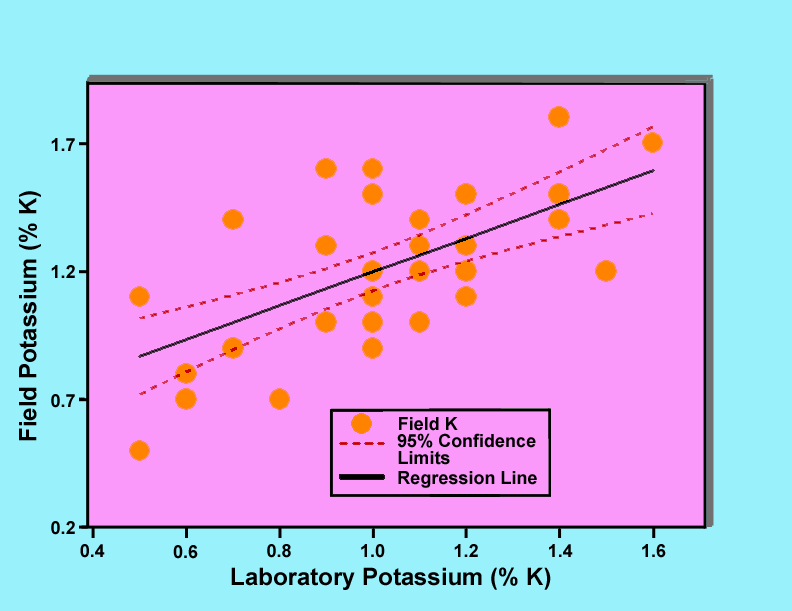
Figure 4. Graph of field gamma-ray determinations of potassium concentrations versus laboratory measurements of samples collected at the locations of the gamma-ray measurements.
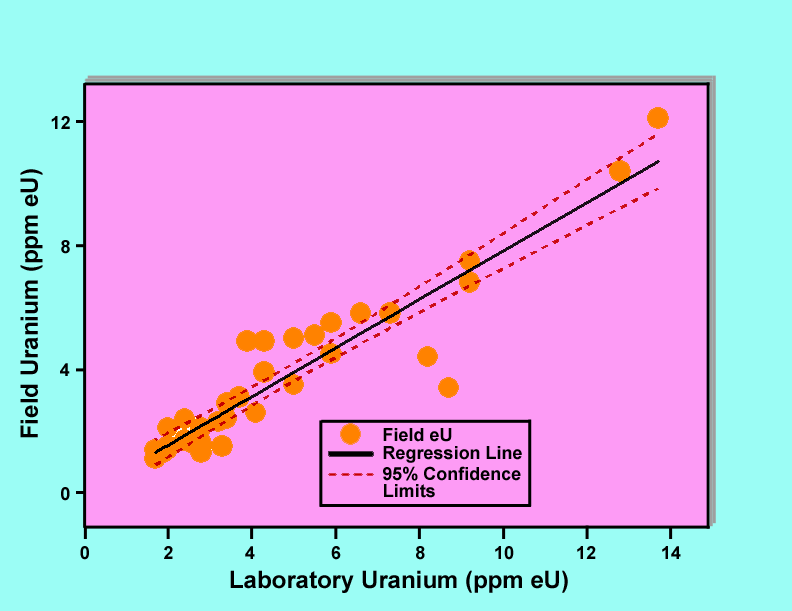
Figure 5. Graph of field gamma-ray determinations of uranium concentrations versus laboratory measurements of samples collected at the locations of the gamma-ray measurements.
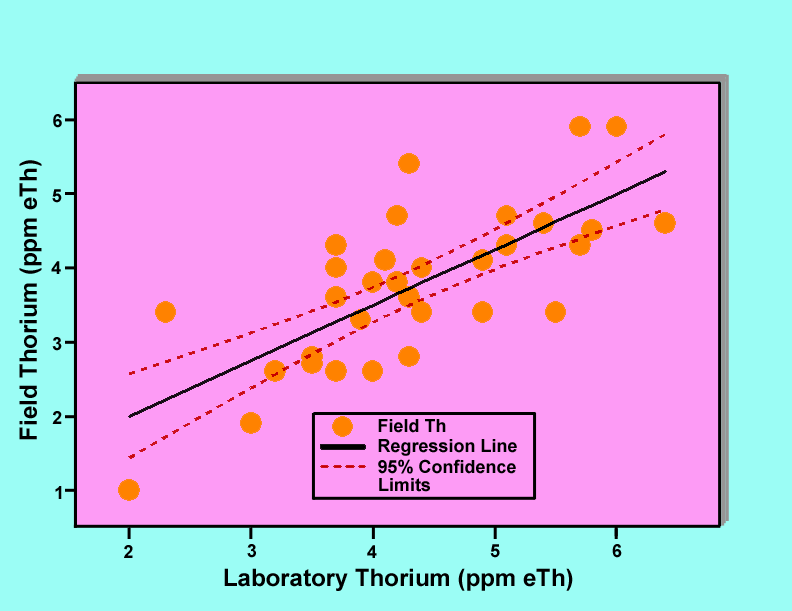
Figure 6. Graph of field gamma-ray determinations of thorium concentrations versus laboratory measurements of samples collected at the locations of the gamma-ray measurements.
Bulk density and soil moisture measurements of the samples were made by packing the samples into tubes of known volumes and measuring the weights of the samples in the tubes. The samples were then dried at a temperature of 110 oC for a period of at least 8 hours. After drying, the samples were weighed again to determine weight loss due to evaporation of water from the samples. The dry samples were then packed into tubes and weighed to obtain the bulk dry density (weight divided by volume). The amount of material that can be packed into the tubes is dependent upon the force applied and we tried to use a consistent amount of pressure applied by hand. The porosity is a calculated quantity that assumes that the density of the soil particles is 2.65 g/cm3. Valdosta State University provides a brief discussion of soil bulk density and porosity. The bulk densities that we have measured are not intended to represent in situ soil properties; however, they do provide a measure of relative porosities of the soils.
The equation used to calculate the porosity is
P = 1 - BD/2.65
where P is porosity and BD is the bulk dry density.
[an error occurred while processing this directive]