 |
|
|
||||
| Open-File Report 01-0429: World Trade Center USGS Bulk Chemistry Results |
| About USGS / Science Topics / Maps, Products & Publications / Education / FAQ |
Representative splits of samples of dusts from the WTC area and of steel girder coatings from the WTC debris were analyzed for total major element composition by Wavelength-Dispersive X-Ray Fluorescence (WD-XRF), for total major and trace element composition by Inductively Coupled-Plasma Mass Spectrometry (ICP-MS), for total carbon and sulfur by combustion, and for carbonate carbon by coulometric titration.
Representative sub-samples of the dust and beam-coating samples were obtained using a standard cone and quartering approach. The sub-samples were then ground and homogenized prior to chemical analysis.
X-ray fluorescence analytical methods, which are summarized in Arbogast (1996), involve heating the sample at 925°C for 40 minutes to determine the weight of volatiles lost from the sample. The sample is then mixed with lithium tetraborate and fused for 56 minutes at 1120°C. The molten sample is poured into a mold and cooled, producing a homogeneous glass disc of the sample material. This disc is then analyzed in a WD-XRF spectrometer, and results are given in total weight percent. The amounts of volatile components in a sample, such as organic material (wood, paper, plastic, other organic compounds), water, sulfur, inorganic carbon, and nitrogen compounds, can be determined by the weight lost by the sample during its initial heating.
ICP-MS analysis was carried out on splits of the samples that were first dissolved using a multi-acid digestion combining hydrochloric, hydrofluoric, nitric, and perchloric acids (Crock et al.,1999, and references therein). ICP-MS methods are given in Briggs and Meier (1999), and Crock et al. (1999).
Total carbon and total sulfur were analyzed by combustion (Crock et al., 1999). Carbonate carbon was determined by coulometric titration, and organic carbon determined by the the difference between total carbon and carbonate carbon (Crock et al., 1999).
Analytical results are tabulated in Chemistry Table 1, and summarized graphically in Chemistry Figures 1-4. The elements measured by the chemical analyses are those routinely measured by the USGS for studies of rocks, sediments, soils, and environmental samples. Total mercury concentrations have not been measured in the WTC solid samples, but have been measured in leach solutions derived from the samples (see the next section of this report). Quality-assurance, quality control data and information for the analyses are available upon request.
These analytical methods determine the total concentration (in weight percent or parts per million) of each element in any given sample. The samples are likely to contain a mixture of different components, such as particles of gypsum, concrete, steel, etc., that together make up the total concentration of elements.
 Larger 141 KB image
Larger 141 KB image
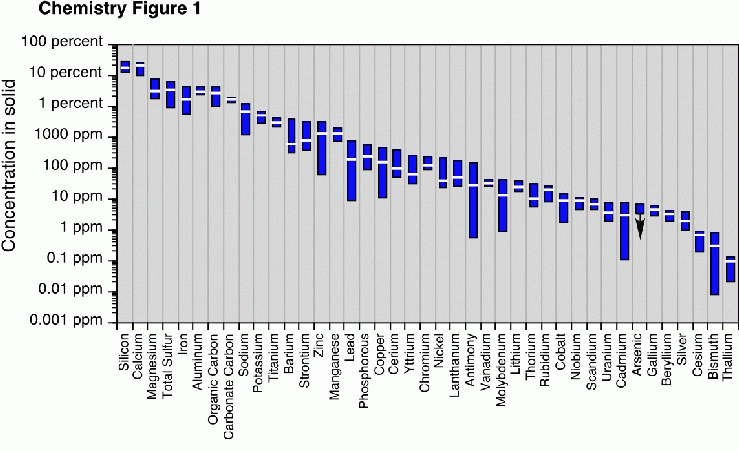
Plot showing the concentration ranges (colored boxes) and means (horizontal white bars) for major and trace elements in samples of WTC dusts and girder coatings. Several samples had arsenic concentrations below the analytical detection limits, indicated on the graph by the arrow extending downward from the detection limit concentration. Concentrations of some elements (such as tin) were not determined n these samples. For comparison, 1 percent equals 10,000 parts per million.
Map of lower Manhattan showing (as stacked bar charts) variations in concentration (in percent) of major elemental components of WTC dust and girder coating samples. Dust samples collected indoors are indicated by the single hatch pattern and girder coating samples by the cross-hatch pattern; all others are dust samples collected outdoors. For comparison with Chemistry Figure 4, 1 percent equals 10,000 parts per million.
Map of lower Manhattan showing (as stacked bar charts) variations in concentration (in percent) of inorganic carbon, carbonate carbon, and total sulfur of WTC dust and girder coating samples. Dust samples collected indoors are indicated by the single hatch pattern and girder coating samples by the cross-hatch pattern; all others are dust samples collected outdoors. For comparison with Chemistry Figure 4, 1 percent equals 10,000 parts per million.
Map of lower Manhattan showing (as stacked bar charts) variations in concentration (in parts per million) of some predominant trace elements of WTC dust and girder coating samples. Dust samples collected indoors are indicated by the single hatch pattern and girder coating samples by the cross-hatch pattern; all others are dust samples collected outdoors. For comparison with Chemistry Figures 2 and 3, 1 percent equals 10,000 parts per million.
WD-XRF results show that silicon, calcium, sulfur, magnesium, aluminum, iron, and carbon are the predominant elemental components of the dusts. The contents of volatile compounds in the dusts approach nearly 20% by weight. The identities and amounts of volatile components (such as water bonded in minerals and adsorbed onto materials; organic materials such as papers, plastics, etc.) in the samples have not been determined. The WD-XRF analyst noted a smell of burning wood or paper in all of the dust samples during sample combustion, another indication that organic material is present in the dusts.
There are no systematic differences in total element composition between the dust samples collected indoors and outdoors, nor are there systematic spatial variations in dust composition between sample sites.
The two samples of girder coating material have generally similar major-element concentrations to those in the dust samples. One notable exception is magnesium, which is somewhat elevated in the girder coating sample (WTC01-08) that has higher chrysotile asbestos content (as determined by SEM analysis).
The dust and girder coating samples are substantially more variable in their trace element compositions than in their major element compositions. In most dust samples, zinc is the predominant trace metal, with concentrations as high as 3000 parts per million. With the exception of one sample that is high in barium (WTC01-16), the trace metals barium, lead, copper, and chromium are present in concentrations of hundreds of parts per million. Concentrations of other trace metals and metalloids such as molybdenum, antimony, and titanium, are tens of parts per million or less. As with the major elements, there are no discernible differences in trace metal content between the dust samples collected outdoors and those collected indoors. There are also no apparent spatial variations in trace-element composition between sample sites.
The girder coating materials (samples WTC01-08, and -09) contain quite low concentrations of trace metals relative to the dust samples.
The total element compositions of the dust samples reflect the chemical makeup of materials such as: glass fibers (containing silicon, aluminum, calcium, magnesium, sodium, and other elements); gypsum (containing calcium and sulfate); concrete and aggregate (containing calcium and aluminum hydroxides, and a variety of silicate minerals containing silicon, calcium, potassium, sodium, and magnesium); particles rich in iron, aluminum, titanium, and other metals that might be used in building construction; and particles of other components, such as computers, etc. Organic carbon in the dusts is most likely from paper, wallboard binder, and other organic materials.
The trace metal compositions of the dust and girder coatings likely reflect contributions of material from a wide variety of sources. Possibilities include metals that might be found as pigments in paints (such as titanium, molybdenum, lead, and iron), or metals that occur as traces in, or as major components of, wallboard, concrete, aggregate, copper piping, electrical wiring, and computer equipment. Further detailed SEM studies of dust and beam coating samples are needed to develop a better understanding of the residences of metals in the samples. A detailed review of the materials used in construction, and the elemental composition of materials commonly found in office buildings would also be useful to understand more completely the potential sources and compositions of the materials in the dusts.
It is important to note that the total chemical analyses presented in this section do not provide an indication of the metals in the dusts and girder coating materials that may potentially be bioavailable (readily assimilated by organisms). For example, heavy metals, such as lead, may occur in forms that range from highly soluble to highly insoluble in water or body fluids. Consequently, high concentrations of total lead in dust samples may or may not translate into elevated concentrations of readily bioavailable lead. Chemical leach tests such as those presented in the next section of this study aid in understanding potential release and bioavailability of heavy metals and other constituents from the girder coatings and dust samples.
NEXT Section of Report: Leachate Studies
Back to document Table of Contents
Contacts
For further information on sub-sampling and sample preparation methods,
contact:
Steve Wilson,
swilson@usgs.gov
For further information on XRF methods, contact:
Joe Taggart,
jtaggart@usgs.gov
For further information on ICP-MS analytical methods, contact:
Paul Lamothe,
plamothe@usgs.gov
For further information on results and interpretation, contact:
Geoff Plumlee,
gplumlee@usgs.gov
| AccessibilityFOIAPrivacyPolicies and Notices | |
| |
|