Open-File Report 01-415
Methods
Results
Discussion
A diverse, well-preserved molluscan fauna was examined from three samples in the Biloxi Formation, Belle Fontaine No. 1 Corehole, Jackson County, Mississippi. A biostratigraphic analysis using the Jaccard similarity coefficient compared the Biloxi to Quaternary deposits from the Atlantic and Gulf Coastal Plains, and peninsular Florida. The results reveal strong affinities between the molluscan fauna of the Biloxi samples and three late middle to late Pleistocene units from South Carolina: the Socastee Formation and informal units Q2 and Q3 of McCartan (1990). A comparison of the Biloxi fauna with stratigraphically important molluscs identified by Blackwelder (1981) indicates the Biloxi was deposited in mollusc zones M2 and M1 (late middle to late Pleistocene and Holocene). The evidence supports assignment of a late middle to late Pleistocene age to the Biloxi Formation. An examination of extant molluscan species present in the samples from the Biloxi Formation indicates it was deposited in a shallow, nearshore, open-marine environment.
A stratigraphic test hole drilled on the Gulf Coast in Jackson County, Mississippi recovered 22.5 feet (6.85 m) of the fossiliferous Biloxi Formation. Otvos (1982, 1991) states that the Biloxi Formation was deposited in a near-shore open marine to brackish environment during the Sangamon interglacial high-stand of sea level. Laterally traceable in the subsurface, the Biloxi Formation is a significant unit in Gulf Coast stratigraphic studies because it crosses the entire northern Gulf Coast (Otvos 1981, 1982, 1991, 1992) and because few marine units were preserved during the subsequent regression of the Wisconsinan glacial stage (Otvos, 1992).
Despite the unique opportunity to study middle to late Pleistocene fossils of the Gulf Coast provided by samples from the Biloxi, no detailed analyses of the well-preserved and diverse molluscan fauna have been conducted. R.H. Parker, H.G. Richards, and B.W. Blackwelder provided a molluscan faunal list for the Biloxi Formation (in Otvos, 1976, appendix). Marsh (1966) included a list of molluscs from a well in Santa Rosa County, Florida panhandle, that he attributed to a marine phase of the Pliocene Citronelle Formation, but that probably represent the Biloxi Formation. In contrast, numerous studies have been done on Holocene molluscs of the Gulf Coast that provide an important source of data for comparison (Vanatta, 1903; Moore, 1961; Hollander and Dockery, 1977; Dockery in Ingram, 1991, appendix III; Andrews, 1977).
This paper provides a stratigraphic and paleoenvironmental analysis of the molluscan fauna recovered from subsurface samples of the Biloxi Formation. The molluscan fauna of the Biloxi is compared to other marine Pleistocene and Holocene deposits of the Gulf and Atlantic coasts. A detailed taxonomic analysis of the molluscs is not within the scope of this paper.
Three samples were examined for molluscs from the USGS Belle Fontaine # 1 core, drilled in 1990 at the west end of Belle Fontaine Beach, Jackson County, Mississippi (Gautier South 7-1/2' quadrangle, 30o20' 43" N, 88o 44' 38" W)(Figure 1); surface elevation of the core is 6 feet and the total core depth is 175'3". Samples 1 (30'6"-31'0") and 2 (32'-32'6") are from the massive sand lithofacies at the top of the Biloxi Formation, and Sample 3 (46'2"-46'5) is from the sand-clay lithofacies at the bottom of the Biloxi Formation (Figure 2).
The samples were washed and picked for molluscs and other macrofauna down to 297 microns. Sample size ranged from 87.5 g (Sample 3) to 197 g (Sample 2) dry-weight. The fauna identified in the three samples are listed in Table 1. The specimens are minute (the majority less than 1 cm) but well preserved (see Plates 1-3 for examples). Diversity is high for such small sample sizes:
|
Samples:
|
Pelecypod Species | Gastropod Species |
|---|---|---|
| 1 | 27 | 20 |
| 2 | 23 | 17 |
| 3 | 22 | 19 |
Data on molluscan occurrences in Pleistocene and Holocene deposits from the Gulf and Atlantic Coastal Plains, and peninsular Florida were compiled from the following sources 1 (Figure 3):
1 Pleistocene stratigraphic units in peninsular Florida are currently under revision (Scott, 1992). The Caloosahatchee, Bermont and Ft. Thompson Formations as originally defined are biostratigraphic units. Formal lithostratigraphic terminology has been proposed by Scott (1992) but not validated. For this paper I am following Scott's usage of the term "faunal unit" to refer to the Caloosahatchee, Bermont, and Ft. Thompson Formations.
| 1) Modern, Gulf of Mexico; Andrews (1977), Dockery in Ingram (1991, appendix 3), Vanatta (1903) |
| 2) Mid-Holocene, Gulf of Mexico, off-shore from Mississippi; Hollander and Dockery (1977) |
| 3) Fort Thompson (transitional) fauna, Florida; Portell, et al. (1992) |
| 4) Bermont and Fort Thompson fauna, Florida; DuBar (1958) |
| 5) Bermont (transitional) fauna, Florida; Portell, et al. (1992) |
| 6) Caloosahatchee fauna, Florida; DuBar (1958), Lyons (1992), Petuch (1982), Ward (1992) |
| 7) Socastee Formation, South Carolina; DuBar, et al. (1980) |
| 8) Canepatch Formation, South Carolina; DuBar, et al. (1980) |
| 9) James City Formation, North Carolina; Ward and Blackwelder (1987) |
| 10) Waccamaw Formation, North and South Carolina; DuBar, et al. (1980) |
| 11) Unit Q1 (informal) South Carolina; Weems and McCartan (1990; based on unpublished faunal lists of B. Blackwelder and L. Ward) |
| 12) Unit Q2 (informal) South Carolina; Weems and McCartan (1990; based on unpublished faunal lists of B. Blackwelder and L. Ward) |
| 13) Unit Q3 (informal) South Carolina; Weems and McCartan (1990; based on unpublished faunal lists of B. Blackwelder and L. Ward) |
| 14) Unit Q4 (informal) South Carolina; Weems and McCartan (1990; based on unpublished faunal lists of B. Blackwelder and L. Ward) |
| 15) Biloxi Formation, Mississippi; this study |
The resulting compiled database contained 2,522 species and 15 stratigraphic divisions. The faunal data were taxonomically standardized so the final data matrix was reduced to 1,216 species; Vaught (1989) served as a guide for standardization of generic and subgeneric names. The faunal lists that were selected for inclusion in the database represented focused, localized molluscan studies where current stratigraphic usage could confidently be applied. The exception is the modern Gulf data set; these data were compiled from studies that ranged from Texas to the Florida panhandle. Comprehensive works such as Dall (1903) were excluded from the analysis because individual collections could not be confidently assigned to a specific stratigraphic division. Published data served as the basis for assigning ages to the units analyzed, but division of the Pleistocene epoch was normalized to conform with the time scale of Berggren et al. (1985).
Using the compiled and standardized database, the Pleistocene stratigraphic units shown in Figure 3 were compared to each other on the basis on the molluscan species they contain by means of the Jaccard similarity coefficient (Tables 2, 3):
Jcij = Nij / (Nij + Ni + Nj)
where Jc is the Jaccard coefficient, Nij is the number of species two localities (i and j) have in common, Ni is the number of species present in locality i but absent in locality j, and Nj is the number of species present in locality j but absent in locality i. As Hazel (1970, p. 3239) explains, the Jaccard coefficient "is simply the proportion of objects in common among the objects present in two units being compared." The Jaccard similarity coefficient was selected because it is a simple binary measure that emphasizes paired occurrences; abundance data, which are more significant for a paleoecological analysis than a biostratigraphic analysis, are excluded. Paired absences, which may or may not be significant biostratigraphically, do not contribute to the calculation of the Jaccard coefficient. A disadvantage of Jaccard, however, is that a high value for Ni or Nj will make the value for the similarity coefficient very small; thus differences in preservation and sampling could skew results. (See Cheetham and Hazel, 1969; Hazel, 1970; and Ward and Gilinsky, 1993, for more detailed discussions on the Jaccard similarity coefficient). The number and percent of species that each unit analyzed had in common with the Biloxi Formation was also examined (Table 4).
The relatively high similarity coefficient values for the Belle Fontaine #1 core samples of the Biloxi Formation (Table 3) indicate the molluscan fauna of the Biloxi is most like that of the informal Q2 and Q3 units of McCartan (1990) and the Socastee Formation of DuBar, et al. (1980) in the late middle to late Pleistocene of South Carolina (Figure 3). The molluscan fauna of the Canepatch Formation in South Carolina (late Pleistocene according to Blackwelder, 1981; middle Pleistocene on the basis of data provided by Bybell, 1990 and using the time scale of Berggren et al., 1985) also have relatively strong affinities to the Biloxi. The Bermont and Fort Thompson faunal units of Florida have moderate molluscan faunal affinities to the Biloxi; the age assignments of these units are currently being debated (see Jones, 1992; and Portell, et al., 1992, for discussion). The James City and Waccamaw Formations (lower Pleistocene; DuBar, et al., 1980; Ward 1992) and the informal Q4 unit (McCartan, 1990) from the Carolinas have relatively low similarity coefficients when paired with the Biloxi. Low molluscan faunal affinities with the Biloxi are also indicated for the mid-Holocene and modern Gulf of Mexico deposits.
| Unit Q2 | 0.1227 |
| Unit Q3 | 0.1196 |
| Socastee Formation | 0.1150 |
| Canepatch Formation | 0.1081 |
| Fort Thompson Faunal Unit | 0.0993 |
| Unit Q5 | 0.0859 |
| Bermont/Fort Thompson Faunal Unit | 0.0827 |
| Bermont Faunal Unit | 0.0744 |
| Mid-Holocene | 0.0722 |
| James City Formation | 0.0622 |
| Waccamaw Formation | 0.0492 |
| Caloosahatchee Faunal Unit | 0.0391 |
| Modern Gulf of Mexico | 0.0353 |
| Unit Q4 | 0.0135 |
| Units: | Percent Species in Common with the Biloxi Formation |
|---|---|
| Unit Q3 | 28.21 |
| Socastee Formation | 20.97 |
| Unit Q2 | 16.81 |
| Fort Thompson Faunal Unit | 15.38 |
| Unit Q5 | 14.47 |
| Bermont/Fort Thompson Faunal Unit | 13.75 |
| Canepatch Formatin | 13.19 |
| Mid-Holocene | 10.08 |
| Bermont Faunal Unit | 9.18 |
| Unit Q4 | 9.09 |
| James City Formation | 8.00 |
| Waccamaw Formation | 5.61 |
| Modern Gulf of Mexico | 4.45 |
| Caloosahatchee Faunal Unit | 4.37 |
A comparison of the ranked similarity coefficients of units paired with the Biloxi Formation (Table 3) to the ranked percent species in common (Table 4) reveals a similar pattern. While th exact order may differ, in general, units with high similarity coefficients when paired with the Biloxi Formation also have a higher percentage of species in common with the Biloxi Formation. Units with low similarity coefficients also have a low percentage of species in common. Unit Q4 is the only exception; it has the lowest similarity coefficient when paired with Biloxi but moves to a more intermediate position when the ranked percentages are examined.
The highest ranked similarity coefficients (Table 3) and the highest ranked percent species in common (Table 4) for the samples from the Biloxi Formation occur when the molluscan fauna of the Biloxi are compared to the fauna of the late middle and late Pleistocene marine units of South Carolina. On the basis of the molluscan biostratigraphy, the Biloxi Formation in the Belle Fontaine No.1 core appears to be late middle to late Pleistocene in age; this age is consistent with deposition during the Sangamon interglacial as proposed by Otvos (1982, 1991).
The age of the South Carolina late middle to late Pleistocene units has been discussed in previous reports. DuBar and DuBar (1980, p. 189) believe the Socastee was deposited during the "Sangamonian glacio-eustatic sea-level rise" of approximately six meters. They correlate the Socastee to the Anastasia, Miami, Key Largo, and Fort Thompson Formations of Florida, and on the basis of radiometric data from the later three units, they date the Socastee at 140 to 120 ka; using the time scale of Beggren, et al. (1985), the Socastee is late Pleistocene. McCartan, et al. (1990) consider units Q2 and Q3 to be late Pleistocene on the basis of the mollusc and ostracode taxa present, uranium-disequilibrium series dates on corals, and amino-acid ratios; several corals from unit Q2 yield an age of 120-90 ka and two corals from unit Q3 yield an age of approximately 200 ka. This numeric age data places nit Q2 in the late Pleistocene, but unit Q3 is late middle Pleistocene on the Berggren, et al. (1985) time scale. Nannofossil recovery in units Q2 and Q3 was limited and could only constrain the units to Pleistocene Zones NN 20 to NN 21a (450 to 75 ka) (Bybell, 1990); NN 21 is latePleistocene and NN 20 is late middle Pleistocene on the Berggren, et al. (1985) time scale. The combined data from South Carolina (DuBar and DuBar, 1980; McCartan et al., 1990) indicate late middle to late Pleistocene deposition of the Socastee Formation, and the informal units Q2 and Q3. The relatively high similarity coefficients for the Biloxi Formation, when paired with these three South Carolina units, and the corresponding high percent species in common for the same three units, suggests the Biloxi was also deposited during the late middle to late Pleistocene.
Further support for a late middle to late Pleistocene age for the Biloxi Formation samples from the Belle Fontaine No. 1 core is provided by a comparison to the molluscan zones developed by Blackwelder (1981). Blackwelder identifies three mollusc zones for the Quaternary of the middle Atlantic Coastal Plain (Figure 4) and lists the ranges of "stratigraphically important molluscan taxa" (Blackwelder, 1981, table 1). Six species identified as important molluscan taxa by Blackwelder occur in the samples examined from Belle Fontaine No. 1 core (Figure 4); all but one of these six species, Noetia limula (Conrad, 1832) (Plate 1, Figure 6), occur in zones M1 and M2, and one of the six species, Anadara ovalis (Bruguiere, 1789) (Plate 2, Figure 8), does not make its first appearance until zone M2. A seventh species, Busycon spiratum (Lamarck), 1816 (also restricted to zones M1 and M2), may be present in sample 1 but it is so minute (less than 4 mm) and poorly preserved that a confident identification is not feasible.
Noetia limula is the only species present in the Biloxi Formation samples examined that indicates deposition before the late middle to late Pleistocene according to Blackwelder's data (1981). There are many possible explanations for the co-occurrence of Anadara ovalis (Zones M1 and M2) and Noetia limula (Zones M3 and M4). The simplest explanation is that I have misidentified the species; I have not examined the type material, but the limited number of specimens I have agree with the original description (Conrad, 1832, p. 29, pl. 1, fig. 1), and with the description and illustrations of Dall (1898, p. 631, pl. 31, fig. 14, 14b) and Ward and Blackwelder (1987, p. 137, pl. 2, fig. 3, 4). Another explanation for the co-occurrence is that Blackwelder's zones are based on middle Atlantic faunal distributions; Noetia limula may have survived longer in the Gulf. A third possibility is that Noetia limula is present above the top of zone M3, but was not seen by Blackwelder; this appears to be the most likely explanation because DuBar, et al. (1980, Table 3) record N. limula from the Canepatch Formation (zone M2). In contrast, Anadara ovalis is only reported from stratigraphic units that correspond to zones M1 and M2 in the literature reviewed. Given the questionable last-occurrence datum for Noetia limula, and the definitive occurrence of Anadara ovalis, I believe the Biloxi samples from Belle Fontaine No.1 core can be confidently assigned to molluscan zones M1 and M2 of Blackwelder, which correspond to late middle to late Pleistocene deposits (upper Pleistocene of Blackwelder's two-part division of the Pleistocene (1981)). Further refinement is impossible with the available molluscan data.
The only upper Pleistocene unit shown on Figure 3 that does not have a relatively high correlation coefficient when paired with the Biloxi samples is the Fort Thompson faunal unit (Table 3), but the age assignments and stratigraphy of the Florida units are in a state of flux (see Scott, 1992; Jones, 1992; and Portell, et al., 1992 for detailed discussions). Recent isotopic data (Jones, 1992) indicates the Fort Thompson faunal unit may be as old as early Pleistocene in age, in which case the relatively moderate correlation coefficient value for the Biloxi-Fort Thompson pair further supports the assignment of the Biloxi to the late middle to late Pleistocene.
Likewise, the relatively intermediate affinities of the Biloxi samples compared to the Canepatch Formation, the combined Bermont and Fort Thompson faunal unit, and the Bermont faunal unit (Table 3, Table 4) support the assignment of the Biloxi to the late middle to late Pleistocene. The Bermont unit is middle Pleistocene at the youngest and possibly as old as 1.6 Ma based on the reversed polarity reported by Jones (1992) from the same Florida locality analyzed by Portell, et al. (1992; source of molluscan data used in calculation of similarity coefficient). The age of the Canepatch Formation in South Carolina is fairly well constrained; 500 to 450 ka ages have been assigned by Blackwelder (1981), DuBar and DuBar (1980), and McCartan, et al. (1990) on the basis of amino-acid dates, uranium-disequilibrium series dates, and faunal content; this data places the Canepatch in the middle Pleistocene using the time scale of Berggren, et al. (1985). An even greater temporal distance is indicated by the relatively low similarity coefficient values for the Biloxi when paired with the early Pleistocene James City and Waccamaw Formations, and the upper Pliocene to lower Pleistocene Caloosahatchee faunal unit.
The relatively low similarity coefficient value for the modern Gulf of Mexico samples when paired with the Biloxi Formation was unexpected because at least 42% of the mollusc species found in the Belle Fontaine samples are extant (Table 1). This low similarity coefficient can be explained by the large difference in the sizes of the two data sets (Table 1: 67 species total for the Biloxi; 427 species total for the modern Gulf). The Jaccard coefficient was selected because it ignores paired absences and so results should not be as skewed by disproportionate sample sizes as they would be if some other coefficients were used, but a high value for Ni or Nj will still lower the similarity coefficient value. In this analysis, the availability of large data sets on modern molluscs from the Gulf, in contrast to the paucity and the localizd nature of the data on the Biloxi, has distorted the outcome.
The ranked positions of unit Q4, unit Q5, and the mid-Holocene similarity coefficients, when paired with the Biloxi Formation, (Table 3) were also unanticipated. The mid-Holocene samples are moderately diverse, compared to the other units included in the analysis (Table 1; 129 species in data set), so the similarity coefficient for the paired mid-Holocene and Biloxi samples may also be affected by relatively high values for Ni or Nj. Alternatively, this may be a valid representation of the similarity of the two units; the molluscan fauna of the Biloxi is more closely allied to late middle and late Pleistocene faunas from other units analyzed than it is to the Holocene fauna. The mid-Holocene samples are also in a medial position when the percent species in common values are ranked. The comparatively high similarity coefficient value for unit Q5 (late early Pleistocene? based on data in McCartan (1990) and using the Berggren, et al. (1985) time scale) when paired with the Biloxi, and the high percent species in common, are more difficult to explain. The diversity recorded in the two data sets is very similar (Table 1; 67 species in the Biloxi data set, 76 species in the unit Q5 data set). Both units were deposited under nearshore conditions, so the similarity coefficient may be detecting similar environments and not similar ages in this case. The lowe similarity coefficient for unit Q4 (Table 3) was especially surprising because Q4 is considered equivalent to the Canepatch Formation (McCartan, 1990; Bybell, 1990) and was therefore expected to have a similar value. However, the percent species in common for unit Q4 and the Canepatch Formation (Table 4) follow the expected pattern; both occupy medial positions on the ranked list. Unit Q4 is the only case where the ranked percent species in common differs significantly from the ranked similarity coefficients.
The general correspondence of the ranked similarity coefficients and the ranked percent species in common indicates the patterns are real, despite a few unexplained results. The bulk of the molluscan biostratigraphic evidence indicates that the samples from the Biloxi Formation at the Belle Fontaine No.1 corehole were deposited during the late middle to late Pleistocene.
The majority of the extant molluscan species present in the samples from the Biloxi Formation at Belle Fontaine No.1 corehole are found in shallow open-marine environments; approximately half of these species occur in water depths of less than 60 feet (Strigilla carnaria Linnaeus, 1758, Chione cancellata (Linnaeus, 1767), Dosinia elegans (Conrad, 1843), Nassarius acutus (Say, 1822), and Turbonilla interrupta (Totten, 1835) for example). Mulinia lateralis (Say, 1822), has a wide salinity tolerance and may indicate proximity to brackish water. Nassarius acutus lives along the shore in the intertidal zone in the modern Gulf. Only one species indicates deposition further out on the continental shelf, Kurtziella cerina ? (Kurtz and Stimpson, 1851), a rare species in Samples 2 and 3, reportedly occurs at depths of 84-1,998 ft. The species present in the three samples examined demonstrate preferences for a variety of habitats (sandy, muddy, grassy, or rocky bottoms); this is consistent with the interpretation of a near-shore environment of deposition for the Biloxi. No significant differences were noted among the three samples. The modern geographic distribution of the extant species and genera present in the samples indicates the climate was very similar to that of the Gulf Coast today.
A biostratigraphic analysis of the well preserved, diverse molluscan fauna in samples from the Biloxi Formation at Belle Fontaine No.1 corehole indicate deposition during the late Pleistocene, possibly the Sangamon interglacial; this conclusion agrees with earlier work (Otvos 1981, 1982, 1991). Two lines of evidence support this age for the Biloxi: 1) relatively high Jaccard similarity coefficients for the Biloxi in pair-groups with the late Pleistocene Socastee Formation and units Q2 and Q3 of McCartan (1990); 2) occurrence of mollusc zone M1 and M2 species (Blackwelder, 1981) in the Biloxi samples. Inaddition, the high percent of extant species (42%) in the Biloxi Formation, and the ranked percent species in common suggest deposition during the later half of the Pleistocene. Modern habitats for extant species found in the Biloxi indicate deposition in a near-shore, shallow, open-marine environment.
Acknowledgments. I want to thank Gregory Gohn (USGS) for bringing these samples to my attention and for our discussions on Pleistocene stratigraphy. David Dockery (Mississippi Department of Environmental Quality, Office of Geology) and Lucy Edwards (USGS) provided valuable comments and suggestions in their reviews of this manuscript.
A number of people have assisted in the technical aspects of preparing this paper. Lauren Hewit (USGS), Marija Balanc (USGS, retired), and Nancy Durika (USGS) helped with the SEM, photography and darkroom work. Computer data entry was done by Lauren Hewit, Jeffrey Stone (USGS NAGT Fellow), Keith Goggin (USGS), and Marija Balanc. Rob Stamm (USGS) prepared the final illustrations and plates.
Andrews, Jean, 1977, Shells and Shores of Texas: Austin, University of Texas Press, 365 p.
Berggren, W.A., Kent, D.V.., Flynn, J.J., and Van Couvering, J.A., 1985, Cenozoic geochronology: Geological Society of America Bulletin: v. 96, p. 1407-1418.
Blackwelder, B.W., 1981, Late Cenozoic stages and molluscan zones of the U.S. middle Atlantic Coastal Plain: Journal of Paleontology, Memoir 12, v. 55, supplement, p. 1-34.
Bybell, L.M., 1990, Calcareous nannofossils from Pliocene and Pleistocene deposits in South Carolina: U.S. Geological Survey Professional Paper, 1367-B, 9 p.
Cheetham, A.H., and Hazel, J.E., 1969, Binary (presence-absence) similarity coefficients: Journal of Paleontology, v. 43, no. 5, p. 1130-1136.
Conrad, T.A., 1832, Fossil Shells of the Tertiary Formations of North America: Philadelphia, v. 1, n. 1, p. 1-20, pls. 1-6 [reprinted by Paleontological Research Institution, Ithaca, N.Y., 1963].
Dall, W.H., 1898, Contributions to the Tertiary fauna of Florida: Transactions of the Wagner Free Institution of Science of Philadelphia, v. 3, part 4, p. 571-947.
_____ 1903, Contributions to the Tertiary Fauna of Florida: Transactions of the Wagner Free Institution of Science of Philadelphia, v. 3, part 6, p. 1219-1654.
DuBar, J.R., 1958, Stratigraphy and paleontology of the late Neogene strata of the Caloosahatchee River area of southern Florida: Florida Geological Survey Bulletin, no. 40, 267 p.
_____, and DuBar S.S., 1980, Neogene biostratigraphy and morphology of northeastern South Carolina, in DuBar, J.R., DuBar, S.S., Ward, L.W., and Blackwelder, L.W., Cenozoic Biostratigraphy of the Carolina outer Coastal Plain: Field Trip No. 9 in Excursions in southeastern geology, vol. 1, R.W. Frey, editor: Geological Society of America, Annual Meeting, p. 179-189.
_____, DuBar S.S., Ward, L.W., and Blackwelder, L.W., 1980, Cenozoic Biostratigraphy of the Carolina outer Coastal Plain: Field Trip No. 9, in Excursions in southeastern geology, vol. 1, R.W. Frey, editor: Geological Society of America, Annual Meeting, p. 211-230.
Hazel, J.E., 1970, Binary coefficients and clustering in biostratigraphy: Geological Society of America Bulletin, v. 81, p. 3237-3252.
Hollander, E.E., and Dockery, D.T., 1977, Molluscan assemblages of the mid-Holocene New Orleans barrier trend: The Compass, v. 55, no. 1, p. 1-29.
Ingram, S.L., 1991, Investigative report on Buoy Reef, western Mississippi Sound, Mississippi: Mississippi Department of Environmental Quality, Office of Geology Open-File Report 14, 70 p [Appendix III, Environmental Evaluation of macrofossils, p. 42-44, compiled by D.T. Dockery.
Jones, D.S., 1992, Integrated stratigraphic approach to geochronology of marine-nonmarine sites in the Plio-Pleistocene of Florida: Florida Geological Survey Special Publication, no. 36, p. 51-62.
Lyons, W.G., 1992, Caloosahatchee-age and younger molluscan assemblages at APAC mine, Sarasota, County, Florida; Florida Geological Survey Special Publication, no. 36, p. 133-159.
Marsh, O.T., 1966, Geology of Escambia and Santa Rosa Counties, western Florida panhandle: Florida Geological Survey Bulletin no. 46, p. 1-140.
McCartan, Lucy, 1990, Studies related to the Charleston, South Carolina, earthquake of 1886 - Neogene and Quaternary lithostratigraphy and biostratigraphy: Introduction to the volume: U.S. Geological Survey Professional Paper, 1367, p. 1-5.
_____, Weems, R.E., and Lemon, E.M., jr., 1990, Quaternary stratigraphy in the vicinity of Charleston, South Carolina, and its relation to local seismicity and regional tectonism: U.S. Geological Survey Professional Paper, 1367-A, 39 p.
Moore, D.R., 1961, The marine and brackish water Mollusca of the state of Mississippi: Gulf Research Reports, v. 1, no. 1, p. 1-58.
Otvos, E.G., Jr., 1976, Post-Miocene development of the Mississippi-Alabama coastal zone: Journal of the Mississippi Academy of Sciences, v. 21, p. 101-114.
_____ 1981, Post-Miocene units, Mississippi-Alabama Coast - a brief outline in Field Trip Guidebook for southern Mississippi, 1981, 30th annual meeting of the southeastern section, Geological Society of America: Southern Geological Society Publication no. 2, p. 1b-1 - 1b-20.
_____ 1982, Santa Rosa Island, Florida Panhandle, origins of a composite barrier island: Southeastern Geology, v. 23, no. 1, p. 15-24.
_____ 1991, Northeastern Gulf Coast Quaternary in Quaternary geology of the Gulf of Mexico Coastal Plain: The Geology of North America, v. K-2, p. 588-594.
_____ 1992, Late Quaternary coastal units and marine cycles: correlations between northern Gulf sectors: American Association of Petroleum Geologists Bulletin, v. 76/9, p. 1465 [abstract].
Petuch, E.J., 1982, Notes on the molluscan paleoecology of the Pinecrest beds at Sarasota, Florida with the description of Pyruella, a stratigraphically important new genus (Gastropoda: Melongenidae): Proceedings of the Academy of Natural Sciences of Philadelphia, v. 134, p. 12-30.
Portell, R.W., Schindler, K.S., and Morgan, G.S., 1992, The Pleistocene molluscan fauna from Leisey shell pit 1, Hillsborough County, Florida: Florida Geological Survey Special Publication, no. 36, p. 181-194.
Scott, T.M., 1992, Coastal Plains stratigraphy: the dichotomy of biostratigraphy and lithostratigraphy - a philosophical approach to an old problem: Florida Geological Survey Special Publication, no. 36, p. 21-25.
Vanatta, E.G., 1903 [1904], A list of shells collected in western Florida and Horn Island, Mississippi: Proceedings of the Academy of Natural Sciences of Philadelphia, v. 55, p. 756-759.
Vaught, K.C., 1989, A classification of the living Mollusca: Melbourne, Florida, American Malacologists, Inc., 189 p.
Ward, L.W., 1992, Diagnostic mollusks from the APAC pit, Sarasota, Florida: Florida Geological Survey Special Publication, no. 36, p. 161-165.
_____, and Blackwelder, B.W., 1987, Late Pliocene and early Pleistocene Mollusca from the James City and Chowan River Formations at the Lee Creek Mine in Geology and Paleontology of the Lee Creek Mine, North Carolina, II: Smithsonian Contributions to Paleobiology, no. 61, p. 113-283.
_____, and Gilinsky, N.L., 1993, Biostratigraphic analysis of the Chowan River Formation (upper Pliocene) and adjoining units, the Moore House Member of the Yorktown Formation (upper Pliocene) and the James City Formation (lower Pleistocene): Virginia Museum of Natural History, Memoir 3, part A, p. 1-29.
Weems, R.E., and McCartan, Lucy, 1990, A summary of selected stratigraphic occurrences of Neogene and Quaternary invertebrate faunas and microfloras in the Charleston, South Carolina, area: U.S. Geological Survey Professional Paper, 1367-G, 31 p.
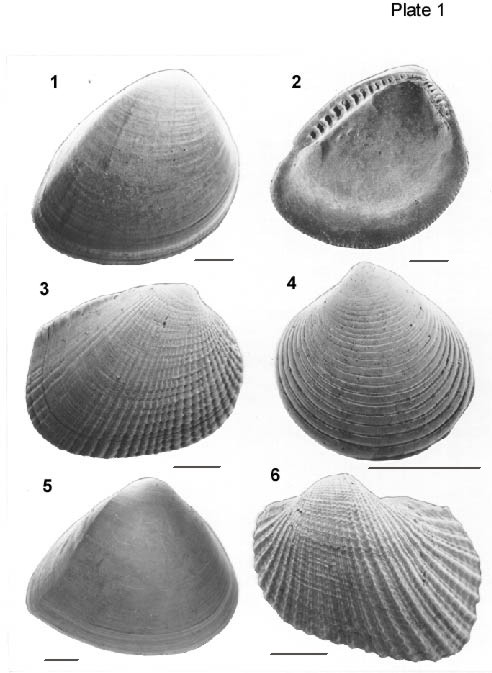
All scale bars are 1 mm.
Figures 1, 2. Nucula proxima Say, 1822.
1. Exterior of left valve from Sample 3, USGS Belle Fontaine #1 Corehole. USNM 484,682.
2. Interior of right valve from Sample 3, USGS Belle Fontaine #1 Corehole. USNM 484,683.
Figure 3. Chione grus (Holmes, 1858). Exterior of right valve from Sample 2, USGS Belle Fontaine #1 Corehole. USNM 484,684.
Figure 4. Dosinia elegans (Conrad, 1843). Exterior of left valve from Sample 1, USGS Belle Fontaine #1 Corehole. USNM 484,685.
Figure 5. Mulinia lateralis (Say, 1822). Exterior of right valve from Sample 2, USGS Belle Fontaine #1 Corehole. USNM 484,686.
Figure 6. Noetia limula (Conrad, 1832). Exterior of left valve from Sample 2, USGS Belle Fontaine #1 Corehole. USNM 484,687.
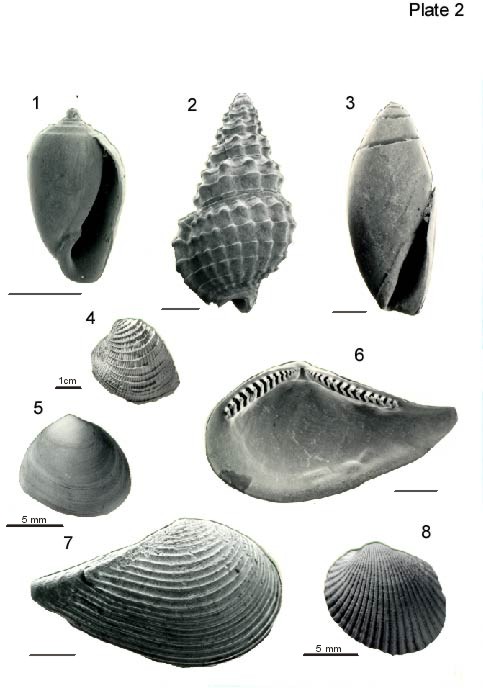
Scale bars are 1 mm unless otherwise indicated.
Figure 1. Acteocina candei (d'Orbigny, 1853). Apertural view from Sample 1, USGS Belle Fontaine #1 Corehole. USNM 484,688.
Figure 2. Nassarius acutus (Say, 1822). Back view from Sample 2, USGS Belle Fontaine #1 Corehole. USNM 484,689.
Figure 3. Olivella sp. Apertural view from Sample 2, USGS Belle Fontaine #1 Corehole. USNM 484,690.
Figure 4. Chione cancellata (Linnaeus, 1767). Exterior left valve from Sample 2, USGS Belle Fontaine #1 Corehole. USNM 484,691. This is the largest specimen found in the Belle Fontaine #1 corehole samples.
Figure 5. Abra aequalis (Say, 1822). Exterior of left valve from Sample 2, USGS Belle Fontaine #1 Corehole. USNM 484,692.
Figures 6, 7. Nuculana acuta (Conrad, 1831).
6. Interior of left valve from Sample 3, USGS Belle Fontaine #1 Corehole. USNM 484,693.
7. Exterior of left valve from Sample 3, USGS Belle Fontaine #1 Corehole. USNM 484,694.
Figure 8. Anadara ovalis (Bruguière, 1789). Exterior of left valve from Sample 3, USGS Belle Fontaine #1 Corehole. USNM 484,695.
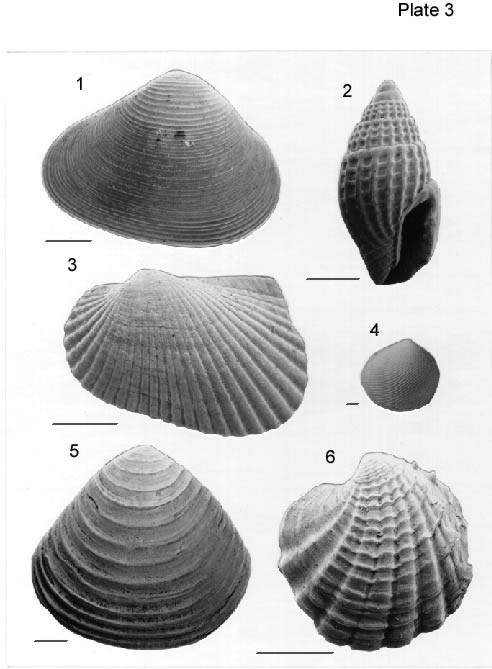
All scale bars are 1 mm.
Figure 1. Ervilia concentrica (Gould, 1862). Exterior of right valve from Sample 1, USGS Belle Fontaine #1 Corehole. USNM 484,696.
Figure 2. Anachis sp. Apertural view from Sample 2, USGS Belle Fontaine #1 Corehole. USNM 484,697.
Figure 3. Anadara transversa (Say, 1822). Exterior view of left valve from Sample 1, USGS Belle Fontaine #1 Corehole. USNM 484,698.
Figure 4. Strigilla carnaria Linnaeus, 1758. Exterior of right valve from Sample 2, USGS Belle Fontaine #1 Corehole. USNM 484,699.
Figure 5. Crassinella lunulata (Conrad, 1834). Exterior of right valve from Sample 1, USGS Belle Fontaine #1 Corehole. USNM 484,700.
Figure 6. Linga amiantus (Dall, 1901). Exterior of right valve from Sample 3, USGS Belle Fontaine #1 Corehole. USNM 484,701.