FIRE and MUD Contents
Anhydrite-Bearing Pumices from the June 15, 1991, Eruption
of Mount Pinatubo: Geochemistry, Mineralogy, and Petrology
By John Fournelle,1 Rebecca Carmody,2 and Arturo S. Daag3
1Department of Earth and Planetary Sciences, Johns Hopkins University, Baltimore, MD 21218. Now at: Department of Geology and Geophysics, University of Wisconsin, 1215 W. Dayton St., Madison, WI 53706.
2U.S. Geological Survey.
3Philippine Institute of Volcanology and Seismology.
ABSTRACT
A distinctive feature of the dacitic pumices from the June 15, 1991, eruption of Mount Pinatubo is the presence of anhydrite, both as microphenocrysts and as inclusions. Over 97 percent of the sulfur present in the dacitic pumice is sulfate. Sulfides are present in minor amounts, mainly as inclusions. Mineral indicators yield the following preeruptive conditions in the dacitic magma: a temperature of 805 to 830° Celsius at a pressure 200-250 megapascals and an oxygen fugacity of 2.5 log units above the Ni-NiO buffer. The presence of cummingtonite rims and groundmass crystals indicates that parts of the magma had cooled to less than 800° Celsius prior to eruption.
Oxygen isotope data indicate that there was no significant contamination of dacitic magma by the underlying Zambales Ophiolite Complex.
Anhydrite is present as inclusions within hornblende and plagioclase crystals; these inclusions confirm the existence of anhydrite in the magma to 9 kilometers in depth. Careful scrutiny of ancient volcanic deposits for anhydrite inclusions may provide evidence for sulfur-rich eruptions in the past even though the anhydrite phenocrysts in these rocks have vanished.
Note to readers: Figures open in separate windows. To return to the text, close the figure's window or bring the text window to the front.
INTRODUCTION
The eruption of Mount Pinatubo on June 15, 1991, produced the largest stratospheric SO2 cloud in recorded history. Approximately 20 Mt were measured by the Total Ozone Mapping Spectrometer aboard the Nimbus-7 satellite (Bluth and others, 1992), nearly three times that of the 1982 eruption of El Chichón. Increasing attention is being paid to such SO2-rich volcanic eruptions, particularly for their influence on global climate.
A major question is the source of the sulfur and its relation to convergent margin volcanism. Experimental studies have shown that significant amounts (thousands of parts per million) of dissolved sulfate can be present in a wet, oxidized silicate melt saturated with anhydrite (CaSO4) (Carroll and Rutherford, 1987; Luhr, 1990).
It generally has been accepted that the sulfur ultimately comes from subducted sulfide-bearing oceanic crust, although this belief does not preclude some possible involvement of crustal sulfates or sulfides. The presence of mafic xenocrysts in pumice at Pinatubo led Fournelle (1991) to suggest possible involvement of underlying Zambales ophiolitic rocks, a suggestion similar to that by Arculus and others (1983) for ophiolite contamination of Mount Lamington (Papua New Guinea) anhydrite-bearing trachybasalts and andesites.
REGIONAL SETTING: THE ZAMBALES RANGE
Mount Pinatubo is located south of the Masinloc massif of the Zambales Range, and east of the Cabangan massif. The Zambales Range has been recognized to consist of ophiolitic fragments, dipping to the east; several areas have been examined in detail (Geary and Kay, 1983; Abrajano and others, 1989; Evans and Hawkins, 1989). Abrajano and Pasteris (1989) described two distinct sulfide associations present in the Acoje massif farther to the north.
A geothermal well slant-drilled from the north flank of Mount Pinatubo encountered mafic material at 1 km in depth, as did another well on its east flank (Delfin and others, this volume). Rocks from the ophiolite complex include microdiorite and diabase dikes, completely altered rocks, hornfels, basalts and gabbros, and monzodiorites extending from ~1.1 to ~2.7 km below the surface (Santos and Diomampo, National Institute of Geological Sciences, Univ. of the Philippines, unpub. report, 1992).
SAMPLE DESCRIPTIONS
Three pumices from the eruption have been examined. Two samples, P1 and P2, were collected from the upper surface of pyroclastic-flow deposits in the Sacobia River valley near Clark Air Base 1 month after the June 15 eruption. These have been studied by several researchers and labeled, respectively, type 2 and type 1 pumices by Imai and others (1993). They also have been cataloged at the Smithsonian Institution: USNM# 116534-1 and 116534-2 (Luhr and Melson, this volume).
Sample P1 is a dark-gray, crystal-poor pumice with cataclastic features, whereas P2 is a white pumice with more (~20 vol%) crystals. A third sample (P4), collected from a pumice-fall layer atop a roof in Angeles City at 1930 on June 15 (Rosalinda M. Temprosa, ICLARM, written commun., 1993), has ~20 vol% of both light- and dark-colored crystals.
Samples of the hybrid andesite (CN6791d) and a basalt inclusion (CN6791i) were examined only for their oxygen isotope compositions. Their major and trace element compositions are reported elsewhere (Pallister and others, this volume).
Several other samples have been examined also. P3 is a Holocene sample from Porac, Pampanga. In hand sample it resembles P2, with ~20 vol% crystals (mostly plagioclase and amphibole).
ZAM1 is a sample of the nearby Zambales Range country rock, from a location 19 km northwest of the Mount Pinatubo crater, near the Balin Baquero River. This area is mapped as "peridotite" by Abrajano and others (1989). This rock is dark, coarse grained, and cut by thin light-green veins.
WHOLE-ROCK CHEMICAL COMPOSITIONS: MAJOR AND TRACE ELEMENTS
Major and minor element whole-rock compositions of these samples were determined by X-ray fluorescence (XRF) analysis at the Department of Geosciences, Franklin and Marshall College, Lancaster, Pa. Major element contents were determined by using the standard LiBO4 fused disk method. Trace element (including sulfur) compositions were determined by the pressed pellet method. Ferrous iron was determined by standard wet chemical method, and loss on ignition (LOI) by heating to 900°C.
Monosulfides (chalcopyrite, sphalerite, pyrrhotite) and disulfides (pyrite) were extracted with hot 6 N HCl and HCl-CrCl2 solution, respectively. The H2S produced was bubbled through a AgNO3 trap and the sulfide collected as Ag2S.
The major, minor, and trace element compositions are given in table 1. Pumices P1, P2, P3, and P4 are dacitic (64-65 wt% SiO2) with 4.4-4.7 wt% Na2O and 1.5-1.6 wt% K2O. They are medium-K calc-alkaline in Miyashiro's (1974) classification.
Table 1. Pinatubo whole-rock chemical analyses.
[ICLARM, International Center for Living Aquatic Resources Management, Manila; LOI, loss on ignition. Major and minor trace element values are in weight percent; trace element values are in parts per million. NA, not applicable]
|
Source
|
Pinatubo
|
Pinatubo
|
Pinatubo
|
Pinatubo
|
Pinatubo
|
Zambales
|
|
Sample
|
P1
|
P2
|
P4
|
P4
|
P3
|
ZAM1
|
|
Type
|
pumice
|
pumice
|
pumice
|
glass separate
|
pumice
|
gabbro
|
|
Eruption date
|
6/15/91
|
6/15/91
|
6/15/91
|
6/15/91
|
Holocene
|
NA
|
|
Location
|
Sacobia River
|
Sacobia River
|
Angeles
|
Angeles
|
Porac
|
Balin Baquero
|
|
Date collected
|
mid-July
|
mid-July
|
6/15/91
|
6/15/91
|
early May
|
unknown
|
|
Collected by
|
C. Newhall
|
C. Newhall
|
ICLARM
|
ICLARM
|
C. Newhall
|
A. Daag
|
|
SiO2
|
64.72 |
63.76 |
65.24 |
75.94 |
64.74 |
49.47 |
|
TiO2
|
.46 |
.52 |
.52 |
.15 |
.52 |
.37 |
|
Al2O3
|
16.43 |
16.11 |
15.64 |
13.99 |
16.05 |
14.97 |
|
Fe2O3
|
1.97 |
2.15 |
2.28 |
.33 |
2.06 |
2.37 |
|
FeO
|
2.03 |
2.18 |
2.25 |
.54 |
2.24 |
5.76 |
|
FeO (total)
|
3.80 |
4.11 |
4.31 |
.83 |
4.09 |
7.89 |
|
MnO
|
.10 |
.10 |
.10 |
.04 |
.10 |
.16 |
|
MgO
|
2.25 |
2.39 |
2.31 |
.29 |
2.35 |
10.39 |
|
CaO
|
4.98 |
5.03 |
4.70 |
1.89 |
4.68 |
13.89 |
|
Na2O
|
4.69 |
4.52 |
4.49 |
4.14 |
4.42 |
1.17 |
|
K2O
|
1.52 |
1.53 |
1.64 |
2.79 |
1.61 |
.02 |
|
P2O5
|
.17 |
.16 |
.18 |
.08 |
.18 |
.00 |
|
LOI
|
.94 |
1.44 |
1.07 |
.76 |
1.98 |
1.91 |
|
Total
|
100.25 |
99.88 |
100.42 |
100.94 |
100.91 |
100.48 |
|
S (ppm)
|
480 |
1,200* |
910 |
170 |
70 |
715** |
|
SO3
|
.120 |
.300 |
.228 |
.042 |
.018 |
.179 |
|
Ba
|
501 |
527 |
566 |
749 |
533 |
10 |
|
Cr
|
66 |
60 |
53 |
19 |
68 |
76 |
|
Ni
|
23 |
17 |
19 |
4 |
21 |
95 |
|
Rb
|
45 |
47 |
52 |
86 |
50 |
6 |
|
Sr
|
561 |
550 |
519 |
345 |
530 |
94 |
|
V
|
89 |
91 |
90 |
11 |
96 |
251 |
|
Y
|
11 |
13 |
12 |
<1 |
12 |
19 |
|
Zr
|
95 |
84 |
104 |
52 |
100 |
47 |
For the large-ion lithophile elements (LILE's), P1-P4 have 501-556 ppm Ba, 519-561 ppm Sr, and 45-52 ppm Rb; for compatible trace elements, Cr is ~60 ppm, Ni ~20 ppm, V ~90 ppm; and for the incompatibles, Y is ~12 ppm and Zr 84-104 ppm.
Whole-rock sulfur contents are highest in the two crystal-rich pumices, 1,200 ppm (P2) and 910 ppm (P4), and low in crystal-poor P1, at 480 ppm. All but one of these samples were collected a month after the eruption. During this time they were exposed to abundant rainwater, and some anhydrite originally present may have dissolved. Anhydrite also may have broken down during deuteric reactions with magmatic water. These values therefore represent minimal sulfur compositions. They are lower than those reported by others, such as 1,500-2,400 ppm (Bernard and others, 1991; Pallister and others, this volume).
One sample, P4, was collected immediately after the eruption; its sulfur content (910 ppm) should not reflect anhydrite dissolution by meteoric water. The differences of sulfur content between the various Pinatubo samples reported in this volume may also reflect some differences in the distribution of anhydrite within the magma.
The Holocene P3 sample contained only 70 ppm S, as might be expected, given the dissolution of anhydrite by meteoric water.
The Zambales country rock is a gabbro with an Mg# (molar Mg x 100/Mg + Fe) of 70. It is moderately oxidized, with 30% of the iron being ferric, and contains 236 ppm monosulfide and 479 ppm disulfide. It is depleted in alkali elements (K, Ba, Rb), and Sr, Y, and Zr are low, whereas Cr and Ni are relatively high.
STABLE ISOTOPES
Whole-rock powders of four Pinatubo dacitic pumices (P1-P4), dome hybrid andesite (CN6791d) and its basalt inclusion (CN6791i), and Zambales Ophiolite Complex sample (ZAM1) were analyzed for their oxygen isotope compositions (fig. 1; table 2). Analyses were performed at the U.S. Geological Survey in Reston, Va. The technique for extraction of oxygen from whole-rock powders follows that of Clayton and Mayeda (1963) with some modifications, including use of ClF3 reagent. Oxygen isotope ratios were measured on a Finnigan MAT 251 mass spectrometer.
Figure 1. Histogram of oxygen isotope compositions for whole-rock powders of Pinatubo and Zambales samples.
Table 2. Pinatubo and Zambales stable isotope compositions.
[Values are in per mil]
|
Sample no.
|
P1
|
P2
|
P4
|
CN6791i
|
CN6791d
|
P3
|
ZAM1
|
|
Type
|
dacite pumice
|
dacite pumice
|
dacite pumice
|
basalt inclusion
|
hybrid andesite
|
dacite pumice
|
ophiolite
|
|
Eruption
|
6/15/91
|
6/15/91
|
6/15/91
|
6/7-12/91
|
6/7-12/91
|
Holocene
|
|
|
Ave. 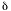 18O 18O
|
7.1 |
7.4 |
7.4 |
6.9 |
7.3 |
7.0 |
4.6 |
|
Ave. 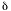 34S 34S
|
n.a. |
8.2 (sulfate) |
n.a. |
n.a. |
n.a. |
n.a. |
1.0 (monosulfide)
0.9 (disulfide) |
Ag2S precipitates from ZAM1 monosulfide and disulfide were combined with Cu2O and combusted at 1,050°C. Sulfate was leached from sample P2 with HCl and precipitated as BaSO4. The BaSO4 was combined with Cu2O and silica glass powder and combusted at 1,150°C. SO2 produced from both Ag2S and BaSO4 was purified of CO2, H2O, and O2 by vacuum distillation. Sulfur isotope ratios were measured on a 6-in., 60°-sector Nuclide Corporation mass spectrometer.
Isotope compositions are reported in the usual 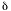 notation in per mil (
notation in per mil ( ) relative to the standard mean ocean water (SMOW) standard for oxygen isotope ratios and relative to the Canyon Diablo troilite (CDT) standard for sulfur isotope ratios. Oxygen and sulfur isotope compositions were measured on two (10-15 mg) aliquots of each sample. Estimated uncertainty on these measurements is +-0.25 and +-0.20 for oxygen and sulfur isotope values respectively.
) relative to the standard mean ocean water (SMOW) standard for oxygen isotope ratios and relative to the Canyon Diablo troilite (CDT) standard for sulfur isotope ratios. Oxygen and sulfur isotope compositions were measured on two (10-15 mg) aliquots of each sample. Estimated uncertainty on these measurements is +-0.25 and +-0.20 for oxygen and sulfur isotope values respectively.
The oxygen isotope compositions of the seven analyzed samples are included in table 2 and shown in figure 1. The most obvious contrast is between the Pinatubo samples, which cluster in the range of 6.9 to 7.4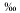 , and the Zambales Ophiolite Complex sample at 4.6
, and the Zambales Ophiolite Complex sample at 4.6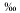 . This lower value falls within the range of analyses (2.3 to 5.9
. This lower value falls within the range of analyses (2.3 to 5.9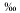 ) of Zambales samples reported by Sturchio and others (1989). The Pinatubo pumices are enriched in 18O relative to the average midocean ridge basalt (MORB)
) of Zambales samples reported by Sturchio and others (1989). The Pinatubo pumices are enriched in 18O relative to the average midocean ridge basalt (MORB) 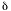 18O of 5.6
18O of 5.6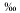 , whereas the Zambales sample is depleted in 18O relative to MORB, probably due to oxygen isotope exchange with meteoric water (Sturchio and others, 1989). This contrast between the Pinatubo pumices and the Zambales Ophiolite Complex suggests that the Pinatubo magma passed through the Zambales Ophiolite Complex without exchanging or assimilating oxygen from it. The Pinatubo dacitic pumice
, whereas the Zambales sample is depleted in 18O relative to MORB, probably due to oxygen isotope exchange with meteoric water (Sturchio and others, 1989). This contrast between the Pinatubo pumices and the Zambales Ophiolite Complex suggests that the Pinatubo magma passed through the Zambales Ophiolite Complex without exchanging or assimilating oxygen from it. The Pinatubo dacitic pumice 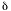 18O values overlap the anhydrite-bearing 1982 El Chichón trachyandesite
18O values overlap the anhydrite-bearing 1982 El Chichón trachyandesite 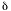 18O of 7.2 to 8.1
18O of 7.2 to 8.1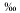 (Rye and others, 1984).
(Rye and others, 1984).
Among the Pinatubo samples, the basaltic inclusion (CN6791i) is the least enriched in 18O, whereas dacitic pumices P2 and P4 are the most enriched. The data in table 2 suggest a subtle contrast in 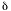 18O between the phenocryst-rich type 1 pumice samples, P2 and P4, and the cataclastic, phenocryst-poor type 2 pumice, P1, with the type 1 pumices being ~0.3
18O between the phenocryst-rich type 1 pumice samples, P2 and P4, and the cataclastic, phenocryst-poor type 2 pumice, P1, with the type 1 pumices being ~0.3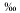 heavier than the type 2 pumice. We believe that this contrast is real, although it should be confirmed by additional oxygen isotope analyses on other type 1 and type 2 samples (the 0.3
heavier than the type 2 pumice. We believe that this contrast is real, although it should be confirmed by additional oxygen isotope analyses on other type 1 and type 2 samples (the 0.3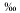 contrast observed is only slightly larger than the analytical uncertainty). This difference could have resulted from an additional boiling event suffered by the type 2 (phenocryst-poor) magma that was not experienced by the type 1 (phenocryst-rich) magma. Pallister and others (this volume) indicate that such a boiling event could account for the abundance of broken crystals in the phenocryst-poor magma. Removal of additional water from the type 2 magma relative to the type 1 magma would cause a slight depletion of 18O in the type 2 magma because a water-rich fluid phase concentrates 18O relative to feldspars and presumably silicic melt (O'Neil and Taylor, 1967) at the preeruptive temperature of 805-830°C (see below).
contrast observed is only slightly larger than the analytical uncertainty). This difference could have resulted from an additional boiling event suffered by the type 2 (phenocryst-poor) magma that was not experienced by the type 1 (phenocryst-rich) magma. Pallister and others (this volume) indicate that such a boiling event could account for the abundance of broken crystals in the phenocryst-poor magma. Removal of additional water from the type 2 magma relative to the type 1 magma would cause a slight depletion of 18O in the type 2 magma because a water-rich fluid phase concentrates 18O relative to feldspars and presumably silicic melt (O'Neil and Taylor, 1967) at the preeruptive temperature of 805-830°C (see below).
The oxygen isotope data also support the hypothesis that the hybrid andesite (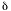 18O=7.3
18O=7.3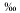 ) represents a mixture of basalt similar to the inclusion CN6791i (
) represents a mixture of basalt similar to the inclusion CN6791i (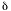 18O=6.9
18O=6.9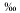 ) and dacitic magma; we would further add that the dacite may have been the heavier (
) and dacitic magma; we would further add that the dacite may have been the heavier (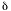 18O=7.4
18O=7.4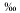 ) type 1 magma.
) type 1 magma.
SULFUR ISOTOPES
Sulfur isotope composition (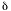 34S) of monosulfides and disulfide from the Zambales Ophiolite Complex sample ZAM1 are 1.0
34S) of monosulfides and disulfide from the Zambales Ophiolite Complex sample ZAM1 are 1.0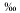 and 0.9
and 0.9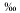 , respectively. These values are comparable, although slightly lighter than the range of 2 to 3
, respectively. These values are comparable, although slightly lighter than the range of 2 to 3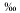 found by Abrajano and Pasteris (1989) in their study of the Acoje critical zone of the Zambales. The sulfur isotope composition of sulfide from the Zambales Ophiolite Complex is slightly heavier than that of Pinatubo chalcopyrite analyzed by McKibben and others (1992, this volume) and McKibben and Eldridge (1993), which gave sulfur isotope compositions in the range of -2 to 9
found by Abrajano and Pasteris (1989) in their study of the Acoje critical zone of the Zambales. The sulfur isotope composition of sulfide from the Zambales Ophiolite Complex is slightly heavier than that of Pinatubo chalcopyrite analyzed by McKibben and others (1992, this volume) and McKibben and Eldridge (1993), which gave sulfur isotope compositions in the range of -2 to 9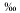 . As expected, sulfate in the Pinatubo pumices is more enriched in 34S than the sulfides with
. As expected, sulfate in the Pinatubo pumices is more enriched in 34S than the sulfides with 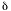 34S values of ~7 to 9
34S values of ~7 to 9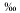 reported by McKibben and Eldridge (1993) and Imai and others (1993) and is in agreement with an analysis of
reported by McKibben and Eldridge (1993) and Imai and others (1993) and is in agreement with an analysis of 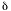 34S=8.2
34S=8.2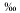 for sulfate from pumice sample P2.
for sulfate from pumice sample P2.
PETROGRAPHY--MINERAL AND GLASS CHEMISTRY
Mineral and glass compositions of the 1992 pumices were determined by using two electron microprobes: a JEOL 8600 in the Department of Earth and Planetary Sciences of the Johns Hopkins University, Baltimore, Md., and a Cameca SX50 in the Department of Geology and Geophysics at the University of Wisconsin, Madison. Operating conditions for both were a 15-keV accelerating voltage with a 20-nA beam current, using a rastered beam for glass analyses and a fixed beam for minerals. Data was reduced with the CITZAF matrix correction program (Armstrong, 1988) for the 8600 and the PAP 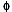 (
(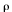 z) routine for the SX50. Representative compositions of silicate and oxide minerals and glasses are given in table 3 and compositions of sulfides in table 4.
z) routine for the SX50. Representative compositions of silicate and oxide minerals and glasses are given in table 3 and compositions of sulfides in table 4.
Table 3. Pinatubo mineral and glass compositions.
[Plag, plagioclase; Hb, hornblende; Bi, biotite; Cum, cummingtonite; Opx, orthopyroxene; Cpx, clinopyroxene; Ol, olivine; Ap, apatite; Mt, magnetite; Ilm, ilmenite; xl, crystal; mega, megacryst; gmass, groundmass; pheno, phenocryst; n.a., not applicable; n.d., not determined. Analytical conditions described in text. Analysis of fluorine done without pulse height discrimination, so values in biotite and apatite are higher than actually present]
|
Sample
|
P2
|
P2
|
P2
|
P2
|
P2
|
P4
|
P4
|
P1
|
P1
|
P2
|
P2
|
P4
|
P2
|
P4
|
P4
|
P1
|
|
Mineral description
|
Matrix glass
|
Melt inclusion
|
Plag with CaSO4
|
Plag with CaSO4
|
Plag
gmass
|
Hb pheno with melt inclusions
|
Hb pheno with melt inclusions
|
Hb
gmass
|
Cum
gmass
|
Cum edge of clot
|
Opx euhedral xls in clot
|
Ap gmass on Mt
|
Ap with CaSO4 (fig. 6C)
|
Mt
with t17
|
Ilm
with t16
|
Ilm
gmass
|
|
ID
|
IX.9-42
|
IX.26-16
|
IX.3-26
|
IX.3-28
|
IX.3-41
|
X.21-t3
|
X.21-t35
|
IX.3-t18
|
IX.3-51
|
IX.3-25
|
IX.3-10
|
X.21-14
|
IX.9-37
|
X.21-t16
|
X.21-t17
|
IX.3-t16
|
|
SiO2
|
78.55 |
74.84 |
59.76 |
54.70 |
60.60 |
45.44 |
48.20 |
47.43 |
55.95 |
54.57 |
55.86 |
0.00 |
0.13 |
0.11 |
0.09 |
0.00 |
|
TiO2
|
.00 |
.00 |
.02 |
.06 |
.02 |
1.86 |
1.02 |
.83 |
.17 |
.25 |
.10 |
.00 |
.00 |
3.89 |
28.52 |
31.18 |
|
Al2O3
|
12.99 |
13.06 |
25.95 |
28.90 |
25.63 |
12.26 |
8.67 |
8.02 |
1.23 |
1.44 |
1.79 |
.00 |
.02 |
1.99 |
.41 |
.33 |
|
Cr2O3
|
.07 |
.00 |
.07 |
.00 |
.01 |
.08 |
.07 |
.03 |
.07 |
.00 |
.00 |
.00 |
.00 |
.19 |
.08 |
.00 |
|
FeO
|
.64 |
1.27 |
.18 |
.22 |
.27 |
8.64 |
12.74 |
13.28 |
16.99 |
17.84 |
10.49 |
.83 |
.10 |
86.14 |
62.75 |
62.12 |
|
MnO
|
.02 |
.15 |
.02 |
.01 |
.00 |
.26 |
.56 |
.57 |
1.12 |
.87 |
.10 |
.00 |
.14 |
.59 |
.24 |
.38 |
|
MgO
|
.20 |
.22 |
.00 |
.03 |
.00 |
15.86 |
14.82 |
14.43 |
21.98 |
21.39 |
31.80 |
.00 |
.09 |
1.2 |
1.27 |
1.31 |
|
CaO
|
1.08 |
1.35 |
7.66 |
11.00 |
7.22 |
11.68 |
10.76 |
10.67 |
1.63 |
1.27 |
.72 |
56.03 |
53.71 |
.04 |
.08 |
.04 |
|
Na2O
|
3.57 |
3.80 |
6.90 |
5.23 |
6.42 |
2.05 |
1.45 |
1.39 |
.16 |
.22 |
.04 |
.00 |
.09 |
.07 |
.11 |
.03 |
|
K2O
|
2.60 |
2.81 |
.31 |
.16 |
.32 |
.52 |
.10 |
.14 |
.18 |
.00 |
.00 |
.00 |
.01 |
.00 |
.00 |
.00 |
|
P2O5
|
.00 |
.07 |
n.d. |
n.d. |
n.d. |
.04 |
.14 |
n.d. |
n.d. |
n.d. |
n.d. |
42.17 |
42.74 |
n.d. |
n.d. |
n.d. |
|
SO3
|
.00 |
.01 |
n.d. |
n.d. |
n.d. |
.27 |
.06 |
n.d. |
n.d. |
n.d. |
n.d. |
n.d. |
.04 |
n.d. |
n.d. |
n.d. |
|
Cl
|
.11 |
.13 |
n.d. |
n.d. |
n.d. |
.00 |
.01 |
n.d. |
n.d. |
n.d. |
n.d. |
.25 |
1.47 |
n.d. |
n.d. |
n.d. |
|
F
|
.00 |
.00 |
n.d. |
n.d. |
n.d. |
n.d. |
n.d. |
n.d. |
n.d. |
n.d. |
n.d. |
2.25 |
1.94 |
n.d. |
n.d. |
n.d. |
|
Total
|
99.81 |
97.68 |
100.87 |
100.31 |
100.49 |
98.85 |
98.57 |
96.79 |
99.48 |
97.85 |
100.90 |
101.53 |
99.29 |
94.53 |
93.66 |
95.39 |
|
Mg#
|
36 |
24 |
n.a. |
n.a. |
n.a. |
77 |
67 |
66 |
70 |
68 |
86 |
n.a. |
n.a. |
n.a. |
n.a. |
n.a. |
|
An#
|
n.a. |
n.a. |
38 |
54 |
38 |
n.a. |
n.a. |
n.a. |
n.a. |
n.a. |
n.a. |
n.a. |
n.a. |
n.a. |
n.a. |
n.a. |
Table 4. Pinatubo sulfide compositions.
[iss, Cu-Fe-S intermediate solid solution; mss, solid solution between Fe1-xS and Ni1-xS; Mt, magnetite]
|
Sample
|
P4
|
P4
|
P2
|
P2
|
P2
|
P2
|
|
Sulfide (phase)
|
CuFeS (iss)
|
CuFeS (iss)
|
(Fe,Ni)S (mss)
|
(Fe,Ni)S (mss)
|
CuFeS (cubanite)
|
(Fe,Ni)S (mss)
|
|
Host
|
Mt
|
Mt
|
Bronzite
|
Bronzite
|
Bronzite
|
Bronzite
|
|
Size, association
|
15 mm
|
15 mm
|
with Mt
|
with Mt
|
with Mt
|
with Mt
|
|
Figure 10 label
|
|
|
P2a 1
|
P2a 2
|
P2a 3
|
P2a 4
|
|
S
|
32.97 |
32.74 |
37.28 |
34.91 |
35.67 |
39.12 |
|
Fe
|
35.80 |
34.38 |
47.07 |
54.04 |
37.38 |
53.40 |
|
Ni
|
.09 |
.10 |
13.91 |
8.14 |
1.22 |
8.21 |
|
Cu
|
30.66 |
32.54 |
.07 |
.26 |
24.78 |
.28 |
|
Total
|
99.52 |
99.76 |
98.33 |
97.35 |
99.05 |
101.01 |
These pumices contain phenocrysts or microphenocrysts (in estimated decreasing abundance) of plagioclase, hornblende, ilmenite, magnetite, biotite, cummingtonite, anhydrite, apatite, orthopyroxene, quartz, and zircon.
Phenocryst-poor (type 2) pumice sample P1 consists mainly of glass and crystal shards, with few phenocrysts of plagioclase, hornblende (some with anhydrite inclusions), Fe-Ti oxides, cummingtonite, quartz, and biotite. No anhydrite microphenocrysts were found in the two thin sections examined.
Phenocryst-rich (type 1) pumice P2 contains plagioclase and hornblende phenocrysts; anhydrite is present both as microphenocrysts (~250 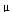 m) and as inclusions in plagioclase and hornblende. Biotite, cummingtonite, Fe-Ti oxides, apatite, quartz, zircon, and bronzite are also present. Sample P4 contains plagioclase, hornblende, biotite, Fe-Ti oxides, anhydrite, apatite, and quartz.
m) and as inclusions in plagioclase and hornblende. Biotite, cummingtonite, Fe-Ti oxides, apatite, quartz, zircon, and bronzite are also present. Sample P4 contains plagioclase, hornblende, biotite, Fe-Ti oxides, anhydrite, apatite, and quartz.
Plagioclase is the dominant phenocryst in the dacitic pumices and ranges in composition from An32 to An55, with most in the range An35-40 (table 3; fig. 2). Hornblende, cummingtonite, biotite, and pyroxenes have Mg numbers ranging from 64 to 77, with the mean ~70 (table 3; fig. 3). Bronzite of Mg# 86 is also present.
Figure 2. Histogram of plagioclase anorthite content. Top, all plagioclase compositions (core, rims, groundmass) shown. Bottom, rims and groundmass compositions plotted; the mean is An36.
Figure 3. Histograms of Mg number of Fe-Mg phases. Top, hornblende, biotite, cummingtonite, and pyroxenes from all samples are plotted. Bottom, hornblendes are plotted by sample number (some cores, rims, and groundmass grains are differentiated).
Groundmass crystals of plagioclase (An40-48), hornblende (Mg# 68), magnetite, and biotite (Mg# 68) are abundant in sample P1. In P2 the groundmass crystals are mainly plagioclase (An34-41), hornblende (Mg# 63-70), and cummingtonite (Mg# 70); initial examination suggests that they are less common than in sample P1.
Some sulfides also were found. Round blebs (<10 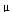 m diameter) of chalcopyrite (Cu-Fe-S intermediate solid solution, iss) are present as inclusions in magnetite and ilmenite in P4 (fig. 4). A variety of sulfides have been found in P2, including FeNiS mss (solid solution between Fe1-xS and Ni1-xS), and cubanite in a complex mafic assemblage. Energy dispersive spectroscopy (EDS) analysis also revealed a 10-
m diameter) of chalcopyrite (Cu-Fe-S intermediate solid solution, iss) are present as inclusions in magnetite and ilmenite in P4 (fig. 4). A variety of sulfides have been found in P2, including FeNiS mss (solid solution between Fe1-xS and Ni1-xS), and cubanite in a complex mafic assemblage. Energy dispersive spectroscopy (EDS) analysis also revealed a 10-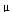 m grain of a Cu-Zn-S phase elsewhere in the matrix glass. Bernard and others (1991) and McKibben and others (this volume) found small amounts of pyrrhotite in Pinatubo pumice.
m grain of a Cu-Zn-S phase elsewhere in the matrix glass. Bernard and others (1991) and McKibben and others (this volume) found small amounts of pyrrhotite in Pinatubo pumice.
Figure 4. Backscattered electron images of P4 sulfide (Cu-Fe-S intermediate solid solution, iss) inclusions in magnetite (A) and ilmenite (B). In A, magnetite is Xusp=0.13, and ilmenite is Xil=0.60. Both oxides are mantled with matrix glass. IL, ilmenite; Mt, magnetite; Ap, apatite; CuFeS, Cu-Fe-S iss.
Matrix and melt inclusion glasses from P2 were analyzed by electron microprobe. The matrix glass is rhyolitic (75-80 wt% SiO2, average = 76.94 wt% for 11 analyses) with low sulfur content (below the detection limit) and ~1,200 ppm Cl. Average Na2O is 3.47 wt%, and K2O is 2.94 wt%. The totals are ~98 to 100 wt%. (See table 3).
Matrix glass was separated from P4 (with heavy liquids and a Frantz magnetic separator) and analyzed by XRF (table 1). It is similar in composition to the rhyolitic matrix glass analyzed by electron microprobe. Its ferric/ferrous content was determined for calculation of melt fO2 state. Trace element contents differ significantly from whole-rock values: Ba (749 ppm) and Rb (86 ppm) are higher than the whole-rock content, whereas lower values are noted for Cr (19 ppm), Ni (4 ppm), Sr (345 ppm), V (11 ppm), Y (<1 ppm), and Zr (52 ppm).
Melt inclusions examined by electron microprobe are slightly lower in SiO2 than matrix glass is (69-77 wt%, average = 73.76 for five analyses), with other oxides as well as sulfur at similar levels to those of the matrix glass (average Na2O = 3.35 wt%, K2O = 2.61 wt%), and having 96 to 97 wt% totals.
Holocene sample P3 contains large (to 2 mm) phenocrysts of plagioclase with healed fractures; hornblende is also present.
Anhydrite and apatite are intimately related frequently (also noted by Bernard and others, 1991). Blocks (200 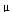 m) of anhydrite and apatite lie side by side in the matrix glass. Apatite inclusions are also present in anhydrite. This anhydrite-apatite association was noted in El Chichón pumices (Luhr and others, 1984).
m) of anhydrite and apatite lie side by side in the matrix glass. Apatite inclusions are also present in anhydrite. This anhydrite-apatite association was noted in El Chichón pumices (Luhr and others, 1984).
ANHYDRITE: WATER DISSOLUTION EXPERIMENT
A simple experiment was performed to determine what type of surface features might be produced in anhydrite by dissolution with water, as possibly might be observed in some of the Pinatubo pumices. Anhydrite (UW collection #8912A, from Bancroft, Ontario) was examined by X-ray diffraction (XRD) to verify its identity; it was then crushed and sieved; 20 to 30 grains of the 1000- to 700-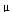 m fraction were put in a beaker with ~125 mL distilled water. The beaker was stationary, unsealed, with no movement of the contents (preventing mechanical breakage). Every 2 to 3 weeks, the water was decanted (some had evaporated), with fresh distilled water added. At the end of 2 months, the water was decanted, and the grains were air dried and mounted for examination. A control group was briefly cleaned ultrasonically in alcohol to remove fragments from surfaces and was then air dried and mounted.
m fraction were put in a beaker with ~125 mL distilled water. The beaker was stationary, unsealed, with no movement of the contents (preventing mechanical breakage). Every 2 to 3 weeks, the water was decanted (some had evaporated), with fresh distilled water added. At the end of 2 months, the water was decanted, and the grains were air dried and mounted for examination. A control group was briefly cleaned ultrasonically in alcohol to remove fragments from surfaces and was then air dried and mounted.
The results of anhydrite sitting in water for 2 months are shown in figure 5B,D with controls in figure 5A,C. The original fractured surfaces (steplike) have remained, with some reduction of their roughness. Notable is the development of distinct, euhedral dissolution pits along the exposed surfaces (fig. 5B). These features resemble both natural and experimental dissolution of feldspars, where selective dissolution occurred in etch pits along apparent dislocations (Berner and Holdren, 1977). On some exposed surfaces (fig. 5D), small acicular crystals are present, possibly gypsum (Hardie, 1967).
Figure 5. Secondary electron images of anhydrite dissolution results after 2 months in distilled water. A and C, Freshly fractured and unreacted surfaces shown. B and D, Dissolution pits are evident in the reacted grains, with tracks of pits marking possible dislocation trails in B (arrowheads). Surface of anhydrite in D contains acicular crystals believed to be gypsum.
We suspect that magmatic dissolution of anhydrite might have similar features present at high surface-energy locations, such as dislocations.
ANHYDRITE IN THE 1991 DACITIC PUMICE
ANHYDRITE INCLUSIONS
Virtually all of the anhydrite trapped within plagioclase and amphibole in sample P2 is rectangular with rounded corners (figs. 6A,B and 7B,D). Similar features were seen in anhydrite in pumice of Nevado del Ruiz (within plagioclase and orthopyroxene; Fournelle, 1990; unpub. data, 1990).
Figure 6. Backscattered electron images showing P2 pumice textures. Anhydrite is present in three forms: as inclusions in plagioclase (A, C), in the matrix glass (B, D), and in intimate relation to apatite (A, B). The anhydrite in D appears to have a round feature in its middle (a core) and to be dissolving along its edges.
Figure 7. Backscattered electron images of anhydrite in P2 pumice. Anhydrite in matrix glass (A, C) has rounded edges, and A in particular is anhedral. Anhydrite in B and D is rounded inclusions in plagioclase. Apatite inclusions in the anhydrite are common. An, anhydrite; Ap, apatite; Hb, hornblende; Gl, matrix glass; Mt, magnetite; Pl, plagioclase.
ANHYDRITE PHENOCRYSTS
The pumices with the highest whole-rock sulfur content also have the greatest abundance of anhydrite microphenocrysts. A mass balance calculation for sample P2 indicates ~0.5 wt% anhydrite in the pumice. The total sulfur content of P2 is 1,200 ppm, with only 27 ppm present as sulfide (4.3 ppm monosulfide, 22.4 disulfide).
Anhydrite in sample P2 has been examined in detail (figs. 6 and 7). Many of the anhydrite phenocrysts in this pumice are euhedral (fig. 6A,C) and have no evidence of dissolution.
Some the anhydrite crystals have irregular edges (fig. 7A), rounded corners (fig. 7C), or features somewhere in between (fig. 6D). Could these anhedral features be indicative of dissolution of the anhydrite?
First, consider the anhydrite in figure 7A, which has one irregular face. This face also has contiguous matrix glass, so deuteric, meteoric, or lab dissolution seems unlikely. The irregular surface, however, resembles the gross features (steplike outline) of the experimentally fractured and then weathered (simulated) anhydrite, although caution must be taken in extrapolating the three-dimensional data (dissolution pits along a surface) to the two dimensions of a crystal surface intersecting the plane of the thin section. This anhydrite from Pinatubo apparently was fractured (along cleavage planes) at some point in its magmatic history; some dissolution is possible, although not certain.
Second, several of the anhydrite crystals have rounded corners, particularly the anhydrite inclusions. We believe these features are not indicative of dissolution, on the basis of the textures of anhydrite in sulfate-water-saturated melt experiments. Luhr (1990) studied sulfate-saturated basalt and trachyandesite at 800 to 1,000°C, 200 to 400 MPa. Anhydrite was present as an equilibrium phase. Backscattered electron (BSE) photomicrographs (his figs. 5 and 6) show that anhydrite at 200 MPa is present in irregular clusters of spherical grains 5 to 10 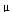 m in size; some oval-shaped grains were also present. At 400 MPa, euhedral prisms and euhedral blades were present as well as spherical grains.
m in size; some oval-shaped grains were also present. At 400 MPa, euhedral prisms and euhedral blades were present as well as spherical grains.
Two anhydrite inclusions are present within hornblende, which is itself within plagioclase (fig. 8A,B). This situation indicates that the anhydrite had been trapped in the dacitic magma at a depth where the hornblende was stable, that is, with a temperature less than ~900°C and minimal melt water content of at least ~2 wt% (Merzbacher and Eggler, 1984; Johnson and Rutherford, 1989a). Plagioclase-bearing anhydrite inclusions in sample P2 range in core composition from An38 to An55, with rims of An38. The occurrence of the plagioclase also sets a maximum temperature range for the hornblende + anhydrite trapping event, given the limited range of plagioclase growth in water-rich melts.
Figure 8. A, B. Backscattered electron images of P2 plagioclase that contains hornblende with an anhydrite inclusion. The aluminum content of this hornblende suggests a pressure of 1.8 to 2.2 kbar (rim-core). Note the ubiquitous apatite in B. C, Hornblende in P2 plagioclase with anhydrite inclusion. D, Hornblende in P1 plagioclase with anhydrite inclusion. An, anhydrite; Ap, apatite; Hb, hornblende; Pl, plagioclase; MI, melt inclusion; Zr, zircon.
SCANNING ELECTRON MICROSCOPE EXAMINATION OF ANHYDRITE
Pumice fragments were glued to a slide, carbon coated, and examined by scanning electron microscope (SEM); no water was involved in the sample preparation. A concentration of a Ca-S phase, presumably CaSO4, was found by EDS on the rim of plagioclase phenocryst (fig. 9). These are apparently growing into a vesicle; no matrix glass is attached to this face of the plagioclase. The hexagonal morphology of these crystals is striking. Anhydrite is ortho-rhombic, although also characterized as pseudohexagonal. The possible CaSO4 hexagonal phases are gamma-CaSO4 or hemihydrate (bassanite), CaSO4 x 1/2H2O (Deer and others, 1962).
Figure 9. Scanning electron microscope photographs of hexagonal Ca-S crystals (indicated by arrows) residing on P2 plagioclase in a coarse pumice fragment. A, Backscattered electron image. B, Secondary electron image. Pl, plagioclase.
There are several possible implications: primary (magmatic) anhydrite may have recrystallized to the hemihydrate polymorph after cooling, with just enough H2O present for the transformation but not enough to dissolve the grain, or these grains may have formed directly from a separate sulfur-rich gas phase. R.B. Symonds (USGS, oral commun., 1992) has described sulfuric acid droplets precipitating on the walls of silica sampling tubes infumaroles at Mount St. Helens. T.M. Gerlach (USGS, oral commun., 1992) has suggested the possibility that sulfuric acid droplets precipitated on plagioclase may have reacted to form the CaSO4.
XENOCRYSTS
Most of the minerals present in the dacitic pumice are consistent with the equilibrium phase assemblages in rhyolitic melt as established by several experimental studies (for example, Johnson and Rutherford, 1989a). Luhr and Melson (this volume), however, present evidence for plagioclase-melt disequilibrium.
Although relatively sparse, the presence of xenocrysts raises the question whether the dacitic magmatic system may have been open to input from other sources.
Hornblende, biotite and cummingtonite in dacite P2 range from Mg# 64 to 73, with most 68 to 70. Present within the thin section are a mat of euhedral (~20 x
50 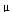 m) bronzite (En84-86) grains surrounded by a ~100-
m) bronzite (En84-86) grains surrounded by a ~100-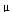 m-wide corona of hornblende and cummingtonite (Mg# 70-73) and magnetite (fig. 10A). Within this bronzite are two ~20-
m-wide corona of hornblende and cummingtonite (Mg# 70-73) and magnetite (fig. 10A). Within this bronzite are two ~20-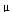 m-wide sulfide grains, Fe-Ni-S and Cu-Fe-S phases, in composite relation to magnetite (fig. 10B,C). Bronzite and Fe-Ni sulfides are uncommon in dacites.
m-wide sulfide grains, Fe-Ni-S and Cu-Fe-S phases, in composite relation to magnetite (fig. 10B,C). Bronzite and Fe-Ni sulfides are uncommon in dacites.
Figure 10. Backscattered electron images of (A) P2 bronzite surrounded by corona of hornblende and iron oxides, with cummingtonite on rim, (B) a closeup of the bronzite showing magnetite-sulfide grains, and (C) a closeup of one magnetite-sulfide intergrowth (MSc). Locations of microprobe analyses of sulfides indicated by nos. 1-4. Cm, cummingtonite; Hb, hornblende; MS, magnetite-sulfide intergrowths; Mt, magnetite, Op, orthopyroxene.
The bronzite and Fe-Ni sulfides suggest interaction of the dacitic magma with either mafic magma or mafic rocks. Two identified candidates are the basaltic magma suggested by Pallister and others (1992) to have triggered the June 1991 eruptions, or the Zambales ophiolite.
GABBRO OF THE ZAMBALES OPHIOLITE COMPLEX
The Zambales sample (ZAM1) consists of calcic plagioclase (An75), clinopyroxene (Mg# 77) and orthopyroxene (Mg# 71), with some ilmenite and magnetite (see table 5 for compositions). Sulfides are also present (table 6): pyrite, pyrrhotite, chalcopyrite, pendlandite, and sphalerite. They exist as complex intergrowths (fig. 11A,B) and as individual grains. Lamellar pyrrhotite is present in clinopyroxene and fills sites of presumably original exsolution lamellae (fig. 11C,D). Secondary silicate minerals are chlorite, edenite-pargasite, subcalcic ferroaugite, and sphene.
Figure 11. Backscattered electron images of sulfides and silicates from Zambales gabbro sample ZAM1. A, B. Sulfides present in An75 plagioclase (Pl) host; complex intergrowth of pyrrhotite (po), pyrite (py), pentlandite (pn), and chalcopyrite (cp). C, D. Clinopyroxene (cpx) with exsolution lamellae replaced with sulfide (po); also replacements of clinopyroxene with edenite-pargasite, subcalcic ferroaugite, and sphene (sp). IL, ilmenite; Am, amphibole.
Table 5. Zambales Ophiolite Complex mineral compositions.
[Plag, plagioclase; Opx, orthopyroxene; Amphib, amphibole; Cpx, clinopyroxene; Mt, magnetite; Ilm, ilmenite; n.a., not applicable]
|
Mineral
|
Plag
|
Cpx
|
Chlorite
|
Opx
|
Amphib
|
Sphene
|
Cpx
|
Ilmenite
|
Magnetite
|
|
ID
|
Ill.10-4
|
Ill.10-5
|
Ill.10-12
|
Ill.10-18
|
Ill.11-8
|
Ill.11-12
|
Ill.11-13
|
Ill.11-20
|
Ill.11-17
|
|
Description
|
with sulfides
|
|
in plag
|
opx
|
edenite lamellae
|
in cpx
|
subcalcic ferroaugite
|
with Mt
|
with Ilm
|
|
SiO2
|
49.76 |
51.71 |
27.42 |
53.98 |
44.98 |
31.78 |
49.48 |
0.03 |
0.14 |
|
TiO2
|
.07 |
.74 |
.03 |
.24 |
2.79 |
32.95 |
.09 |
49.67 |
.41 |
|
Al2O3
|
32.00 |
2.90 |
19.31 |
1.46 |
10.28 |
3.04 |
2.95 |
.10 |
.30 |
|
Cr2O3
|
.00 |
.04 |
.00 |
.03 |
.01 |
.00 |
.00 |
.00 |
.13 |
|
FeO*
|
.22 |
7.63 |
19.74 |
17.89 |
12.58 |
1.56 |
24.60 |
46.66 |
91.67 |
|
MnO
|
.00 |
.20 |
.22 |
.36 |
.15 |
.01 |
1.87 |
2.44 |
.00 |
|
MgO
|
.00 |
14.07 |
18.13 |
24.64 |
13.70 |
.25 |
8.03 |
.06 |
.09 |
|
CaO
|
14.69 |
22.06 |
.16 |
.85 |
11.54 |
27.66 |
9.14 |
.00 |
.05 |
|
Na2O
|
3.01 |
.25 |
.00 |
.02 |
2.15 |
.00 |
.00 |
.07 |
.09 |
|
K2O
|
.03 |
.00 |
.00 |
.00 |
.00 |
.00 |
.00 |
.00 |
.00 |
|
Total
|
99.78 |
99.60 |
85.01 |
99.47 |
98.18 |
97.25 |
96.16 |
99.03 |
92.88 |
|
Mg#
|
n.a. |
77 |
62 |
71 |
66 |
22 |
37 |
n.a. |
n.a. |
|
An#
|
75 |
n.a. |
n.a. |
n.a. |
n.a. |
n.a. |
n.a. |
n.a. |
n.a. |
Table 6. Zambales Ophiolite Complex sulfide compositions.
[n.d., not determined]
|
Mineral
|
Pyrite
|
Chalcopyrite
|
Pentlandite
|
Pyrrhotite
|
Sphalerite
|
|
ID
|
Ill.10-6
|
Ill.10-10
|
Ill.10-12
|
Ill.10-14
|
Ill.11-25
|
|
Description
|
|
|
5 mm
|
|
|
|
Fe
|
48.34 |
32.74 |
31.43 |
61.54 |
12.78 |
|
S
|
54.60 |
34.90 |
33.32 |
39.62 |
33.02 |
|
Cu
|
n.d. |
33.14 |
n.d. |
.20 |
n.d. |
|
Ni
|
n.d. |
n.d. |
32.44 |
.23 |
n.d. |
|
Zn
|
n.d. |
n.d. |
n.d. |
n.d. |
53.81 |
|
Total
|
102.94 |
100.78 |
97.19 |
101.59 |
99.61 |
Pallister and others (1992, this volume) examined basaltic inclusions present in the dacite at Pinatubo and found several mafic phases: Mg-rich olivine (Fo86-89) with Cr-spinels, clinopyroxene (Mg# 69-85), and plagioclase (An20-69).
The bronzite found in dacite pumice P2 is more magnesian (Mg# 86) than the orthopyroxene (Mg# 71) found in the Zambales sample examined here. The bronzite's source remains to be identified.
PREERUPTIVE INTENSIVE PARAMETERS
Minerals present in the June 15 Pinatubo dacitic pumices have been evaluated for evidence of preeruptive magmatic conditions. Adjacent grains of ilmenite (XIlm= .55-.60) and magnetite (XUlsp = .12-.13) from P4 yield temperatures of 805 to 830°C and log fO2 of -11.0 to -11.3 (2.3 to 2.5 log units above the NNO buffer when calculated by using the geothermometer code of Ghiorso and Sack, 1991). These grains pass the Mg/Mn equilibrium Fe-Ti oxide partitioning test (Bacon and Hirschmann, 1988). The fO2 is corroborated by the ferric/ferrous content of separated matrix glass from pumice P4 (table 1), which yields an fO2 of 2 to 3 log units above the NNO buffer when calculated by the model of Kilinic and others (1983).
Rutherford (1991) found cummingtonite rims on Pinatubo hornblende and used them to constrain maximum temperature to 800°C and pressure between 200 and 400 MPa (Geschwind and Rutherford, 1992).
The Al-in-hornblende geobarometer of Johnson and Rutherford (1989b) has been applied to the dacitic pumices, although it is not strictly applicable, given the lack of sphene and alkali feldspar (the K2O level in the Pinatubo dacite is significantly lower than that of the experimental compositions used for their calibration).
P1, P2, and P4 hornblende compositions have been evaluated, and 20 give values ranging from 160 to 360 MPa (fig. 12). All of the hornblendes from dacitic pumice samples P1 and P2 fall in the range from 160 to 260 MPa, with a concentration at 200 to 250 MPa. The Mg# of these hornblendes ranges from 66 to 71.
Figure 12. Histogram of pressures from aluminum contents of Pinatubo hornblendes that is based upon the experimental calibration of Johnson and Rutherford (1989a). Hornblende in dacite pumices P1 and P2 crystallized at pressures between 160 and 260 MPa, with most in range 200 to 250 MPa. There is evidence in P4 hornblendes for somewhat higher pressures, with the maximum near (greater than) the seismically determined base of the magma reservoir.
The P4 pumice contains hornblendes of the similar composition and inferred pressure range, as well as three others giving pressures of 280, 290, and 360 MPa. These contain no gross evidence suggestive of disequilibrium (that is, Mg# <68, no Cr-spinels or oxides).
Three other analyzed hornblende crystals have aluminum contents that would suggest high pressures (310, 450, and 530 MPa--not plotted in fig. 12); however these have Mg# of 68 to 77 and Cr-spinels or other oxides. These latter hornblendes may have formed by reaction of silicic melt with magnesium-rich olivine or bronzite, and equilibrium may not have been achieved with the required silicic mineral assemblage.
Anhydrite exists as inclusions within hornblende (partially enclosed by An39 plagioclase in P2); the hornblende aluminum contents give a core pressure of 220 MPa, with 180 MPa on the rim. Another hornblende with an anhydrite inclusion gives a pressure of 240 to 280 MPa.
The presence of relatively large (to 50 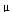 m) oxide inclusions in some hornblende crystals suggests changing conditions in the magma reservoir; hornblende destabilizes with increasing temperature or decreasing XH2O in the melt (Rutherford and Devine, 1988).
m) oxide inclusions in some hornblende crystals suggests changing conditions in the magma reservoir; hornblende destabilizes with increasing temperature or decreasing XH2O in the melt (Rutherford and Devine, 1988).
Cummingtonite, stable only below 800°C (Geschwind and Rutherford, 1992), either rims hornblende or is in the groundmass in the samples examined. The lack of larger cummingtonite crystals suggests that parts of the dacitic magma cooled below 800°C only just prior to eruption.
SULFUR-MAGMA PHASE EQUILIBRIA
With estimates of temperature, pressure, and oxygen fugacity, the Pinatubo dacite may be evaluated in light the experimental phase equilibria studies of Carroll and Rutherford (1987) and Luhr (1990). Their experiments showed that sulfur solubility increased with increasing temperature, pressure, and oxygen fugacity in fluid-saturated anhydrite-bearing andesitic to dacitic magmas. Their data have been plotted in figure 13A, with sulfur solubility versus pressure, for temperatures between 800 and 900°C and for fO2 3 to 5 log units above the NNO buffer.
Figure 13. A, Sulfur solubility in dacitic to rhyolitic melt: concentration in parts per million sulfur versus pressure, for 800-900∞C, fluid-saturated (mostly water), and fO2 ≥3 log units above the NNO buffer. Isotherms based upon the experimental data of Carroll and Rutherford (1987) and Luhr (1990). B, Inferred temperature and pressure field of dacitic magma before cooling below 800∞C and cummingtonite crystallization, as derived from Fe-Ti oxides and Al-hornblende geobarometry.
The Pinatubo melt (glass) compositions are rhyolitic, compared to the andesitic to rhyolitic glass compositions of the experiments (most are dacitic to rhyodacitic). The results are assumed to be similar. Sulfur present in the matrix glass (60 ppm: Westrich and Gerlach, 1992) is consistent with a equilibration pressure of ~150 MPa at 800°C (fig. 13A). Rutherford (1993), Imai and others (1993), and Gerlach and others (this volume) indicate that the Pinatubo melt was at or near water saturation before eruption.
In light of these experimental results, we want to evaluate the question raised by Westrich and Gerlach (1992): is it possible to dissolve in the Pinatubo rhyolitic melt (at the determined T, P, PH2O and fO2) the amount of sulfur inferred from the SO2 released into the atmosphere?
There is generally good agreement on the preeruptive intensive parameters deduced from mineral compositions in the Pinatubo dacites (table 7), although some differences exist, depending upon exactly which timeframe is being considered. One timeframe is that of cummingtonite rim growth immediately preceding eruption, whereas another is that of the host hornblende crystallization some time prior.
Table 7. Summary of temperature, pressure, and oxygen fugacity calculations for the June 15 dacite.
[n.d., not determined]
|
|
Temperature (°C)
|
Pressure (MPa)
|
log fO2
|
|
Rutherford (1991)
|
800+-20
|
225+-50
|
-11.0
|
|
Imai and others (1993)
|
800-850
|
n.d.
|
-11.0 to -11.5
|
|
This study
|
805-830
|
160-280*
|
-11.0 to -11.3
|
|
|
|
170-360**
|
|
|
Pallister and others, this volume
|
776+-22
|
200+-50
|
-11.3
|
According to Westrich and Gerlach (1992), in order to account for the amount of SO2 released into the atmosphere by direct loss from the Pinatubo melt, it would have had to contain ~1,000 ppm S prior to the sulfur's release. Could this much sulfur be dissolved in a rhyolitic melt under the conditions summarized in table 7?
The experimental data from Carroll and Rutherford (1987) and Luhr (1990) plotted in figure 13 suggest that in order to dissolve this much sulfur in the melt, higher temperatures and (or) pressures are required -- for example, 850°C at 500 MPa or 900°C at 250 MPa. An additional constraint is the magma reservoir's depth of 6 to 11 km (seismically determined by Mori and others, 1993), and thus a maximum pressure of ~330 MPa.
Considering the pressure and temperature conditions summarized in table 7, a maximum temperature of 850°C at 330 MPa would permit ~600 ppm S to be dissolved in the melt. For the average pressure (200-250 MPa) and temperature (805-830°C) estimates, the water-rich rhyolitic melt could have held ~400 ppm S (fig. 13B). This is close to the maximum melt-inclusion sulfur content (330 ppm) in hornblende reported by Westrich and Gerlach (1991).
Only, at most, half of the SO2 erupted could then have been theoretically dissolved as sulfate in the melt at the maximum depth and temperature. The discrepancy is larger, for more sulfur must be present in the preeruptive system to account for the anhydrite present in the pumices after eruption, as the anhydrite does not appear to be xenocrystic (McKibben and Eldridge, 1993). Pallister and others (this volume) report an average of 1,200 ppm S from seven analyses of the phenocryst-rich dacite, similar to the results found in this study (P2 and P4). Bernard and others (1991) reported a maximum of 2,210 ppm S in one dacite. This represents mainly anhydrite phenocrysts and inclusions, and these values are minimum because of a possible anhydrite loss during a month of rain on the pumices. It is unlikely that the dacitic magma could have held all this sulfur as dissolved sulfate in the melt. The suggestion of Westrich and Gerlach (1992) and Gerlach and others (this volume) that a volatile phase containing most of the SO2 separated from the melt at depth, prior to melt-inclusion formation, is an attractive hypothesis.
Baker and Rutherford (1992) suggested anhydrite breakdown as possibly being involved in the SO2-rich eruption. The textures of anhydrite in this study support the suggestion by Westrich and Gerlach (1992) and Gerlach and others (this volume) that the SO2 vented to the atmosphere was not significantly augmented by breakdown of anhydrite immediately prior or during the eruption.
CONCLUSION
The June 1991 eruption of Mount Pinatubo presents an opportunity to study sulfur-rich magmas in a convergent-margin volcano. Such eruptions are of increasing concern for their climatic effects. Experimental phase equilibria have shown that oxidized andesitic-dacitic magmas have significant sulfur solubility and that anhydrite is a stable phase in these magmas.
Anhydrite is present in the Pinatubo pumices as microphenocrysts and as inclusions within silicate phenocrysts (hornblende and plagioclase). Assuming the anhydrite is not xenocrystic, its presence within hornblende indicates that the melt was sulfate saturated up to 280 MPa.
Many of the anhydrite microphenocrysts are euhedral. Most of the inclusions, as well as some microphenocrysts, have rounded edges, which we suggest are equilibrium features and not caused by dissolution. Dissolution of a minor amount of anhydrite microphenocrysts, however, cannot be ruled out.
An additional occurrence of anhydrite or a hydrated CaSO4 phase was found in the pumice, possibly formed by crystallization from a gas phase or by reaction of condensed sulfuric acid with plagioclase.
Mineral geothermometers and barometers in the Pinatubo June 15 pumice suggest preeruptive dacitic magma temperatures of 805 to 830°C with fO2 ~2.5 log units above NNO. Assuming the Al-hornblende geobarometer to be valid for these low K2O samples, hornblende crystallized over a range of pressures from 160 to 360 MPa, with most in the range 200 to 250 MPa. Rims and groundmass grains generally give lower values, 160 to 190 MPa. Cummingtonite rims and groundmass grains suggest that the magma had cooled to below 800°C just prior to eruption.
Oxygen isotope data indicate that assimilation of silicate material from the underlying Zambales Ophiolite Complex is insignificant. Assimilation of significant amounts of Zambales sulfide is also unlikely, in light of the sulfur isotope compositions of the Zambales sulfides compared with the Pinatubo dacite sulfate 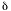 34S.
34S.
Additionally, oxygen isotopic analyses of the two types of June 15 dacite suggest slight differences possibly related to an additional boiling event in the phenocryst-poor dacitic magma. The earlier hybrid andesite could have resulted from a mixture of basalt- and phenocryst-rich dacite.
Phase equilibria suggest that a quantity of sulfur equal to that vented into the stratosphere could not have been dissolved in the melt at 805 to 830°C and 200 to 250 MPa.
Finally, the presence of anhydrite inclusions within other phases is significant and suggests that it may be possible to study these more enduring trapped crystals from old volcanic deposits for evidence of SO2-rich eruptions long after anhydrite phenocrysts have dissolved.
ACKNOWLEDGMENTS
The authors thank Chris Newhall for samples and discussions, Lou Walter (NASA) and Steve Self (University of Hawaii) for encouragement, Tom Moritz (California Academy of Sciences) for sample P4, Steve Sylvester (Franklin and Marshall College) and Maya Wheelock (Johns Hopkins University) for XRF assistance, Amy Gribb (University of Wisconsin-Madison) for XRD work, and the American Geophysical Union for funds to attend the Chapman Conference on Climate, Volcanism, and Global Change. Microprobe analyses were supported by National Science Foundation grants EAR-8916850 and DPP-9117576 to Bruce Marsh of the Johns Hopkins University. Thanks also go to Chris Newhall, Terry Gerlach, James Luhr, and Akira Imai for their reviews, which helped improve the manuscript.
REFERENCES CITED
Abrajano, T.A., Jr., and Pasteris, J.D., 1989, Zambales ophiolite, Philippines, II. Sulfide petrology of the critical zone of the Acoje massif: Contributions to Mineralogy and Petrology, v. 103, p. 64-77.
Abrajano, T.A., Pasteris, J.D., and Bacuta, G.C., 1989, Zambales ophiolite, Philippines, I. Geology and petrology of the critical zone of the Acoje massif: Tectonophysics, v. 168, p. 65-100.
Arculus, R.J., Johnson, R.W., Chappell, B.W., McKee, C.O., and Sakai, H., 1983, Ophiolite-contaminated andesites, trachybasalts, and cognate inclusions of Mount Lamington, Papua New Guinea: Anhydrite-amphibole-bearing lavas and the 1951 cumulodome: Journal of Volcanology and Geothermal Research, v. 18, p. 215-247.
Armstrong, J.T., 1988, Quantitative analysis of silicate and oxide materials: Comparison of Monte Carlo, ZAF, and  (
(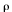 z) procedures, in Newbury, D.E., ed., Microbeam analysis, Proceedings of the 23rd Annual Conference of the Microbeam Analysis Society, August 8-12, 1988: San Francisco, Calif., San Francisco Press, Inc., p. 239-246.
z) procedures, in Newbury, D.E., ed., Microbeam analysis, Proceedings of the 23rd Annual Conference of the Microbeam Analysis Society, August 8-12, 1988: San Francisco, Calif., San Francisco Press, Inc., p. 239-246.
Bacon, C.R., and Hirschmann, M.M., 1988, Mg/Mn partitioning as a test for equilibrium between coexisting Fe-Ti oxides: American Mineralogist, v. 73, p. 57-61.
Baker, L., and Rutherford, M.J., 1992, Anhydrite breakdown as a possible source of excess sulfur in the 1991 Mount Pinatubo eruption [abs.]: Eos, Transactions, American Geophysical Union, v. 73, p. 62 5.
Bernard, A., Demaiffe, D., Mattielli, N., and Punongbayan, R.S., 1991, Anhydrite-bearing pumices from Mount Pinatubo: Further evidence for the existence of sulphur-rich silicic magmas: Nature, v. 354, p. 139-140.
Berner, R.A., and Holdren, G.R., Jr., 1977, Mechanism of feldspar weathering: Some observational evidence: Geology, v. 5, p. 369-372.
Bluth, G.J.S., Doiron, S.D., Schnetzler, C.C., Krueger, A.J., and Walter, L.S., 1992, Global tracking of the SO2 clouds from the June, 1991 Mount Pinatubo eruptions: Geophysical Research Letters, v. 19, no. 2, p. 151-154.
Carroll, M.R., and Rutherford, M.J., 1987, The stability of igneous anhydrite: Experimental results and implications for sulfur behavior in the 1982 El Chichón trachyandesite and other evolved magmas: Journal of Petrology, v. 28, no. 5, p. 781-801.
Clayton, R.N., and Mayeda, T.K., 1963, The use of bromine pentafluoride in the extraction of oxygen from oxides and silicates for isotopic analysis: Geochimica et Cosmochimica Acta, v. 27, p. 43-52.
Deer, W.A., Howie, R.A., and Zussman, J., 1962, Rock-forming minerals: Non-silicates, v. 5: New York, John Wiley, p. 202-225.
Delfin, F.G., Jr., Villarosa, H.G., Layugan, D.B., Clemente, V.C., Candelaria, M.R., Ruaya, J.R., this volume, Geothermal exploration of the pre-1991 Mount Pinatubo hydrothermal system.
Evans, C., and Hawkins, J.W., Jr., 1989, Compositional heterogeneities in upper mantle peridotites from the Zambales Range Ophiolite, Luzon, Philippines: Tectonophysics, v. 168, p. 23-41.
Fournelle, J., 1990, Anhydrite in Nevado del Ruiz November 1985 pumice: Relevance to the sulfur problem: Journal of Volcanology and Geothermal Research, v. 42, p. 189-201.
------1991, Anhydrite and sulfide in pumices from the 15 June 1991 eruption of Mount Pinatubo: initial examination [abs.]: Eos, Transactions, Americal Geophysical Union, v. 72, p. 68.
Geary, E.E., and Kay, R.W., 1983, Petrological and geochemical documentation of ocean floor metamorphism in the Zambales ophiolite, Philippines, in Hayes, D., ed., The tectonic and geologic evolution of Southeast Asian seas and islands, pt. 2: American Geophysical Union, p. 139-156.
Gerlach, T.M., Westrich, H.R., and Symonds, R.B., this volume, Preeruption vapor in magma of the climactic Mount Pinatubo eruption: Source of the giant stratospheric sulfur dioxide cloud.
Geschwind, C.-H., and Rutherford, M.J., 1992, Cummingtonite and the evolution of the Mount St. Helens (Washington) magma system: An experimental study: Geology, v. 20, p. 1011-1014.
Ghiorso, M.S., and Sack, R.O., 1991, Fe-Ti oxide geothermometry: thermodynamic formulation and the estimation of intensive variables in silicic magmas: Contributions to Mineralogy and Petrology, v. 108, p. 485-510.
Hardie, L.A., 1967, The gypsum-anhydrite equilibrium at one atmosphere pressure: American Mineralogist, v. 52, p. 171-200.
Imai, A., Listanco, E.L., and Fujii, T., 1993, Petrologic and sulfur isotopic significance of highly oxidized and sulfur-rich magma of Mt. Pinatubo, Philippines: Geology, v. 21, p. 699-702.
------this volume, Highly oxidized and sulfur-rich dacitic magma of Mount Pinatubo: Implication for metallogenesis of porphyry copper mineralization in the Western Luzon arc.
Johnson, M.C., and Rutherford, M.J., 1989a, Experimentally determined conditions in the Fish Canyon Tuff, Colorado, magma chamber: Journal of Petrology, v. 30, p. 711-737.
------1989b, Experimental calibration of the aluminum-in-hornblende geobarometer with application to Long Valley caldera (California) volcanic rocks: Geology, v. 17, p. 837-841.
Kilinic, A., Carmichael, I.S.E., Rivers, M.L., and Sack, R.O., 1983, Ferric-ferrous ratio of natural silicate liquids equilibrated in air: Contributions to Mineralogy and Petrology, v. 83, p. 136-140.
Luhr, J.F., 1990, Experimental phase relations of water- and sulfur-saturated arc magmas and the 1982 eruptions of El Chichón volcano: Journal of Petrology, v. 31, no. 5, p. 1071-1114.
Luhr, J.F., Carmichael, I.S.E., and Varekamp, J.C., 1984, The 1982 eruptions of El Chichón volcano, Chiapas, Mexico: Mineralogy and petrology of the anhydrite-bearing pumices: Journal of Volcanology and Geothermal Research, v. 23, p. 69-108.
Luhr, J.F., and Melson, W.G., this volume, Mineral and glass compositions in June 15, 1991, pumices: Evidence for dynamic disequilibrium in the dacite of Mount Pinatubo.
McKibben, M.A. and Eldridge, C.S., 1993, Sulfur isotopic systematics of the June 1991 eruption of Mount Pinatubo: a SHRIMP ion microprobe study: Eos, Transactions, American Geophysical Union, v. 74, p. 668.
McKibben, M.A., Eldridge, C.S., and Reyes, A.G., 1992, Multiple origins of anhydrite in Mount Pinatubo pumice: Eos, Transactions, American Geophysical Union, v. 73, p. 633-634.
------this volume, Sulfur isotopic systematics of the June 1991 Mount Pinatubo eruptions: A SHRIMP ion microprobe study.
Merzbacher, C., and Eggler, D.H., 1984, A magmatic geohygrometer: application to Mount St. Helens and other dacitic magmas: Geology, v. 12, p. 587-590.
Miyashiro, A., 1974, Volcanic rock series in island arcs and active continental margins: American Journal of Science, v. 274, p. 321-355.
Mori, J., Eberhart-Phillips, D., and Harlow, D., 1993, 3-Dimensional velocity structure at Mount Pinatubo, Philippines [abs.]: Resolution of magma bodies and earthquake hypocenters: Eos, Transactions, American Geophysical Union, v. 74, p. 667.
O'Neil, J.R., and Taylor, H.P., Jr., 1967, The oxygen isotope and cation exchange of feldspars: American Mineralogist, v. 52, p. 1414-1437.
Pallister, J.S., Hoblitt, R.R., and Reyes, A.G., 1992, A basalt trigger for the 1991 eruptions of Pinatubo volcano?: Nature, v. 356, p. 426-428.
Pallister, J.S., Hoblitt, R.P., Meeker, G.P., Knight, R.J., and Siems, D.F., this volume, Magma mixing at Mount Pinatubo: Petrographic and chemical evidence from the 1991 deposits.
Rutherford, M.J., 1991, Pre-eruption conditions and volatiles in the 1991 Pinatubo magma [abs.]: Eos, Transactions, American Geophysical Union, v. 72, p. 62.
------1993, Experimental petrology applied to volcanic processes [abs.]: Eos, Transactions, American Geophysical Union, v. 74, p. 49-52.
Rutherford, M.J., and Devine, J.D., 1988, The May 18, 1980 eruption of Mount St. Helens, 3. Stability and chemistry of amphibole in the magma chamber: Journal of Geophysical Research, v. 93, p. 11949-11959.
Rye, R.O., Luhr, J.F., and Wasserman, M.D., 1984, Sulfur and oxygen isotope systematics of the 1982 eruptions of El Chichón volcano, Chiapas, Mexico: Journal of Volcanology and Geothermal Research, v. 23, p. 109-123.
Sturchio, N.C., Abrajano, T.A., Jr., Murowchick, J.B., and Muehlenbachs, K., 1989, Serpentinization of the Acoje massif, Zambales ophiolite, Philippines: Hydrogen and oxygen isotope geochemistry: Tectonophysics, v. 168, p. 101-107.
Westrich, H.R., and Gerlach, T.M., 1991, Concentrations of sulfur in Mount Pinatubo glass inclusions [abs.]: Eos, Transactions, American Geophysical Union, v. 72, p. 62.
------1992, Magmatic gas source for the stratospheric SO2 cloud from the June 15, 1991 eruption of Mount Pinatubo: Geology, v. 20, p. 867-870.
FIRE and MUD Contents
PHIVOLCS | University of Washington Press | U.S.Geological Survey
This page is <https://pubs.usgs.gov/pinatubo/four/>
Contact: Chris Newhall
Last updated 06.10.99
![]() 18O
18O ![]() 34S
34S