Determination of Antimycin–A in a Liquid Formulation by High Performance Liquid Chromatography–Mass Spectrometry
Links
- Document: Report (671 KB pdf) , HTML , XML
- Data Release: USGS data release - Data release for determination of antimycin–a in liquid formulation by high performance liquid chromatography–mass spectrometry
- Download citation as: RIS | Dublin Core
Acknowledgments
This project was funded by the U.S. Geological Survey’s Biological Threats and Invasive Species Research Program. We thank several staff from the U.S. Geological Survey for their participation with various stages of planning, setup, and completion of this project.
Abstract
Pesticide formulations containing the active ingredient antimycin–a (ANT–A) have been used by fisheries and aquaculture managers for several decades to remove nuisance fish species. Analytical methods for measuring ANT–A during pesticide treatments have been done using high performance liquid chromatography (HPLC) paired with multiple detection methods (for example, electrochemical, ultraviolet, fluorescence, mass spectrometry). However, instruments and analytical chemistry methods can advance over time because of the need to develop timely, reliable, cost effective, and reproducible methods. Subsequently, ANT–A analytical chemistry methods and sample processing techniques also have improved over the past several decades. In the present study, we describe a liquid chromatography–mass spectrometry method and its verification across three analysts. Each analyst group created a single calibration curve and verified ANT–A in a liquid formulation using the averaged total response of all major ANT–A homologs (A1, A3, A3, A4). The advantage of this technique is that it creates a more resilient ANT–A quantification method amendable to batch-batch differences in major homologs. The method demonstrated how ANT–A can be effectively measured with high accuracy (98–99 percent), precision (2.7–16.2 percent), and specificity within a pesticide liquid formulation using a method applicable for Federal registration requirements.
Introduction
For decades, the global transfer and establishment of species outside their original geographic range has detrimentally affected aquatic biological communities across multiple trophic levels (Kolar and others, 2010; Tricarico and others, 2016). Fish introductions have been particularly pervasive and have sometimes resulted from purposeful actions, such as the creation of food sources or biological control (Fuller and others, 1999). Fisheries and aquaculture managers have frequently relied on pesticide formulations that contain active ingredients, such as rotenone, antimycin–a (ANT–A), and 4-nitro-3-(trifluoromethyl)phenol (TFM; Fredricks and others, 2021) to remove nonindigenous fish. The use of ANT–A for nuisance fish control was reduced for several years after a lapse in pesticide registration. Recently, the U.S. Geological Survey Upper Midwest Environmental Sciences Center (UMESC) obtained the right and possession of Streptomyces sp. (bacteria), which produces a high yield of ANT–A, and is preparing a dossier to reinstate an ANT–A liquid formulation with the U.S. Environmental Protection Agency (EPA). As part of the reinstatement process, a validated analytical method for quantifying ANT–A in the liquid formulation is desired.
The quantification of ANT–A is challenging because, as a product of bacterial fermentation, the chemical consists of multiple homologs and stereoisomers whose abundances vary among batches and manufacturers. The ANT–A chemical structure is shown in figure 1, and table 1 lists the functional groups associated with the four most abundant homologs, which typically account for 80–100 percent of total chemical batch purity. Early attempts to characterize the potency and efficacy of ANT–A relied on fish and yeast bioassays (Lee and others, 1971). Extensive improvements have been made in analytical instrumentation and have led to the quantification of ANT–A in the nanograms per liter range (Bernardy and others, 2013). Bernardy and others (2013) developed a high performance liquid chromatography–mass spectrometry (LC–MS) method capable of quantifying ANT–A in water across multiple water types and sample volumes (50–250 milliliters [mL]) with satisfactory accuracy and precision. This technique quantifies each of the four dominant homologs individually, using their own unique calibration curves, and then estimated total ANT–A concentration by summing the abundances of the four dominant homologs. Although this method was a noteworthy improvement over previous analytical approaches, the Bernardy and others (2013) method has been unable to consistently meet typical data acceptability criteria requiring that the coefficient of determination (r2) of calibration curves be greater than or equal to (≥) 0.995. To address this shortcoming, we have developed a combined response method that performs more effectively, particularly when a reference standard or test material contains homologs that are low in abundance relative to others. This method is intended to meet the EPA Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA; 7 U.S.C. §136 et seq.) requirements for measuring the active ingredient in pesticides. In this report, we present the method used by UMESC scientists and an independent analytical laboratory to demonstrate the specificity, accuracy, and precision for ANT–A quantification in a liquid formulation.
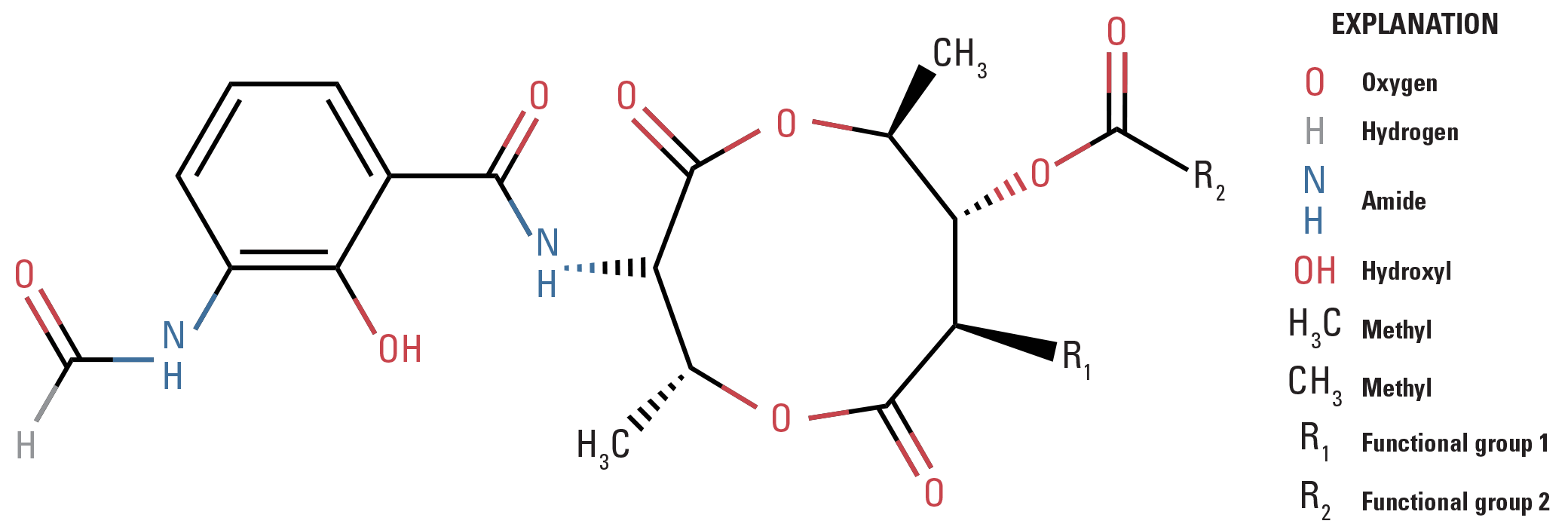
Chemical structure of the antimycin–a analog. R1 and R2 groups vary among homologs.
Materials and Methods
Antimycin–a calibration standards were prepared with the following reagents. Acetonitrile (ACN; Chemical Abstracts Service [CAS]: 75-05-8, Fisher Optima LC–MS Grade, Pittsburgh, Pennsylvania) was dried by adding 2 grams of sodium sulfate (CAS: 7757-82-6, Thermo Fisher, Pittsburgh, Pa.) per 100 mL of solvent. The mixture was swirled and left to sit for a minimum of 30 minutes (min). A primary chemical stock solution was prepared by weighing out ANT–A (CAS 1397-94-0, purity=89.48 percent, Sigma-Aldrich, St. Louis, Missouri) on a Mettler Toledo XPR2 Micro Balance (Mettler-Toledo LLC, Columbus, Ohio) and dissolving the chemical in dried ACN with 1-percent formic acid (ACNFA; CAS 64-18-6, Fisher, Optima LC–MS Grade, Pittsburgh, Pa.). This stock solution was then diluted to create six calibration standards that ranged from 1 to 450 micrograms per liter (µg/L). Standards were stored in 20-mL amber glass screw-top vials that contained 0.2 gram per liter sodium sulfate at −20 degrees Celsius (°C) and were used for as many as 14 days.
Two different solutions (solutions 1 and 2) of liquid ANT–A were prepared. For each solution, solid ANT–A was weighed on a Mettler Toledo XPR2 Micro Balance and dissolved in solvent associated with the previously registered pesticide formulation. Solution 1 was then diluted with ACNFA to generate a sample within the working range of the instrument used in this study (described below). Solution 2 was prepared by combining the dissolved ANT–A sample with inert ingredients associated with the previously registered pesticide formulation and then diluted with 0.1-percent ammonium hydroxide (AMH; CAS 1336-21-6, Sigma-Aldrich, St Louis, Mo.) in dried ACN (AMH–0.1 percent). The AMH–0.1 percent is an important alkalization step to promote optimal binding of ANT–A (solution 2) to the sorbent of a solid phase extraction (SPE) cartridge. Removal of any non-targeted compounds in solution 2 samples was done using an Oasis MAX (1.0 cubic centimeter) SPE cartridge (Waters Corp., Milford, Massachusetts). The SPE cartridges were placed on an extraction vacuum manifold and conditioned with 1.0 mL of methanol (MeOH; CAS No. 67-56-1, VWR, HiPerSolv CHROMANORM, Randor, Pa.), acetone (CAS No. 67-64-1VWR BDH Chemicals, HPLC Grade, Randor, Pa.), and 18.2 megaohm (MΩ) ultrapure water. Solvents were pulled through the SPE cartridges at a flow rate about (~)1.0 milliliter per minute (mL/min) and discarded. Once conditioned, the SPE cartridges were dried by drawing air for 1 min. After drying, 1.0 mL of solution 2 was added to each SPE cartridge. The samples were gravimetrically filtered through the SPE sorbent to facilitate maximum binding and then vacuum was applied to fully draw the solution through the SPE cartridge and dry residual solvent. Elution of ANT–A from the SPE cartridge was achieved gravimetrically using 1.0 mL of ACNFA. Samples were collected into glass test tubes and transferred into LC–MS vials for analytical verification.
The ANT–A concentration in each solution was quantified using an Agilent 1290 Infinity II HPLC (Agilent Technologies, Santa Clara, California) in tandem with an Agilent G6460A triple quadrupole mass spectrometer (QQQ; Agilent Technologies, Santa Clara, Calif.) using an Agilent Jetstream negative mode electrospray ionization as the ion source. Capillary voltage was set to 3500 volts, and sheath gas flow was 10.0 liters per minute at 350 °C. The nebulizer pressure set to 20 pounds per square inch.
High performance liquid chromatography (HPLC) separations were carried out on a Phenomenex Kinetex 1.7 micrometers (µm) particle size, XB-C18 100 Å, 100x2.10 millimeter (mm) column (Phenomenex, Torrance, Calif.). Mobile phase A was composed of 80:20 18.2 MΩ water:MeOH, 0.1-percent formic acid (FA) and 5-millimolar (mM) ammonium acetate (AA; CAS 631-61-8, Millipore Sigma, LiChropur, St. Louis, Mo.). Mobile phase B was composed of 70:30 MeOH:isopropyl alcohol (CAS 67-63-0, HoneyWell, Riedel-de Haën, Charlotte, North Carolina), 0.1-percent FA, and 5-mM AA. The flow rate was 0.3 mL/min for the entirety of the acquisition. The starting acquisition method composition of 50:50 mobile phase A:B transitioned to 100-percent mobile phase B from 0 to 2.15 min, holding at 100-percent mobile phase B from 2.15 to 2.20 min and returning to 50:50 mobile phase A:B from 2.20 to 3.30 min, with a final stop time of 7.0 min.
The peaks associated with four of the most abundant ANT–A homologs (A1, A2, A3, and A4) were used to quantify the total ANT–A concentration in each calibration standard. A single calibration curve was generated by averaging the response of the A1–A4 homologs as a function of known standard concentrations ranging from 1 to 450 μg/L (fig. 2) with the Agilent MassHunter software (Agilent Technologies, Santa Clara, Calif.). The calibration curve was used to determine the total ANT–A concentration in solution 1 and 2.
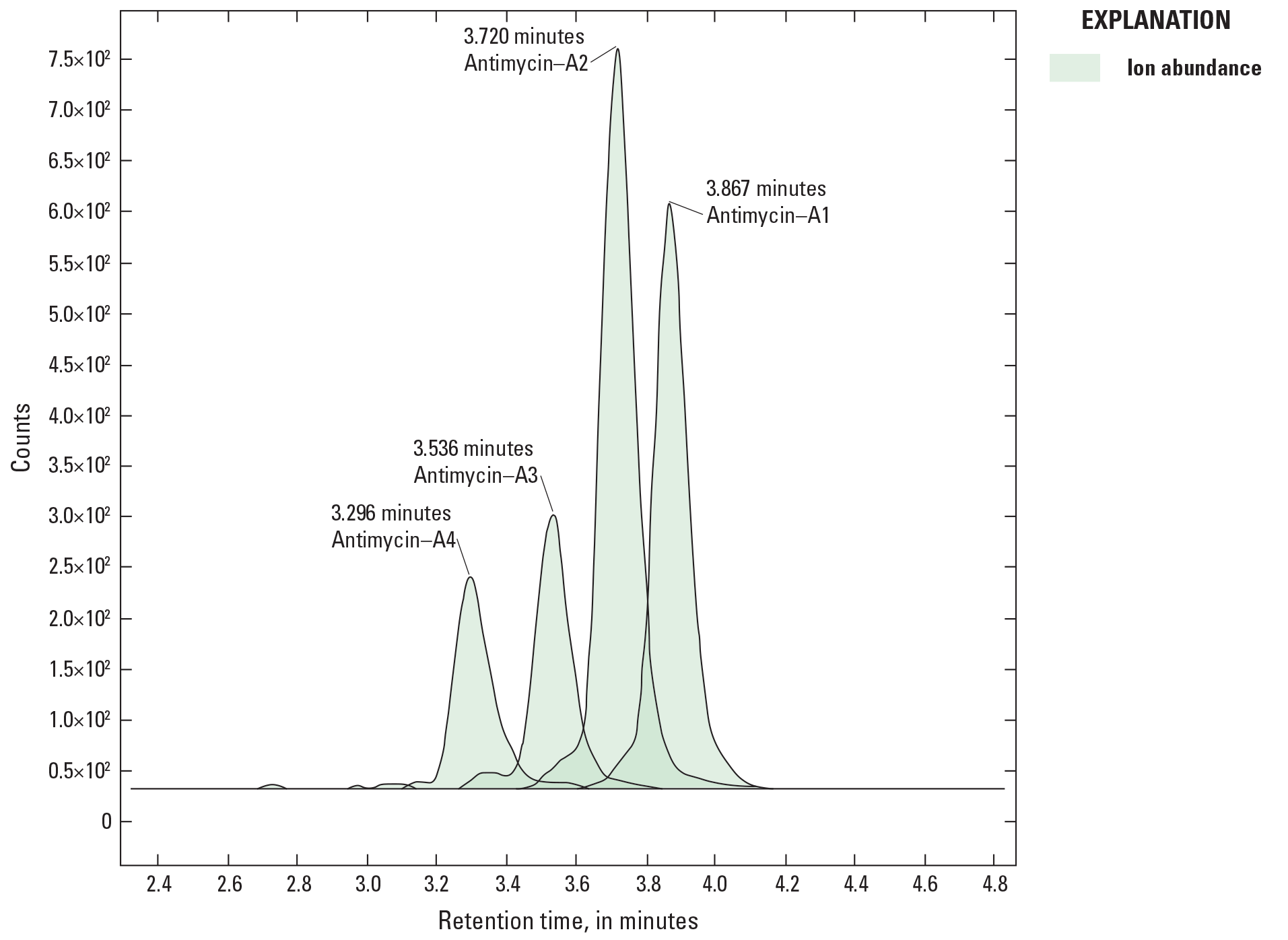
Peaks for four antimycin–a homologs from a single sample injection (for example, 100 micrograms per liter). The plot is normalized ion abundance compared to time (minutes).
Summary statistics (mean concentration, standard deviation, relative standard deviation [RSD] percentage) of single and triple injected samples were calculated to determine if data met acceptability criteria (for example, r2≥0.995, concentration accuracy plus or minus [±] 20 percent, ANT–A concentration precision less than [<] 20-percent RSD) per analyst. The mean (± standard error) and relative standard deviation of the ANT–A concentration and concentration accuracy for solutions 1 and 2 were calculated across three different analysts and acquisitions. One-way analysis of variance (alpha-value less than or equal to [≤] 0.05 significance) was used to compare ANT–A solution concentration accuracies across three separate analysts using SigmaPlot (v14.5; Systat, Inc; San Jose, Calif.).
Results
The four main ANT–A homologs were identified using retention time, precursor ions, and product ions (table 2). Specifically, the confirmatory ions include the combined presence of a precursor ion and all product ions from 191.1 to 263.1 mass to charge ratio (m/z; table 2). The product ion of 245.0 m/z was used as the quantifier for all homologs. The retention times and ion ratios (product ion to precursor response) of the standard and solution 1 and 2 peaks were consistent under the same chromatography conditions.
Table 2.
Antimycin–a ion monitoring parameters of the mass spectrometer for calibration standards and sample solutions.[m/z, mass to charge ratio; µs, microsiemens; V, voltage; Cell Acc, cell accelerator; ANT–A homolog, antimycin–a structural homolog; 245 m/z is the quantifier ion for all analogs and other product ions are for verifying antimycin–a]
The calibration curve was quadratically fit through six standards concentrations ranging from 1 to 450 µg/L. All calibration curves were quadratic and above acceptability criteria (r2≥0.995)
Across three separate acquisitions, the mean (±standard error [SE]) ANT–A concentration accuracy values for solution 1 containing a nominal concentration of 230 µg/L ANT–A were 97.8±1.5 percent, 98.5±5.2 percent, and 98.8±3.2 percent (table 3) and were not significantly different (p-value=0.062). Combining the three solution 1 datasets for all three acquisitions yielded a mean accuracy of 98.4±1.4 percent. Additionally, the mean ANT–A concentration accuracies of solution 2 at a nominal concentration of 100 µg/L were 114.9±10.7 percent, 96.7±8.7 percent, and 84.4±6.4 percent (table 3) and were not significantly different (p=0.966) across three separate acquisitions. When combining the three solution 2 datasets for all three acquisitions, the mean accuracy was 98.7±5.7 percent.
Table 3.
Antimycin–a solution 1 and 2 mean concentrations, mean percentage accuracy, and relative standard deviation per analyst group during the method validation. Data summarized from Saari and others (2024).[Sol-n, solution number; µg/L, microgram per liter; conc., concentration; RSD, relative standard deviation; %, percentage]
The ANT–A concentration precision (RSD percentage) across analysts was between 2.5 and 5.7 percent (solution 1) and 4.8 and 16.7 percent (solution 2; table 3). The accuracy precision across analysts was between 2.7 and 5.6 percent (solution 1) and 7.6 and 16.2 percent (solution 2; table 3). All data associated with this study are available in Saari and others (2024).
Discussion
Natural products from bacterial fermentation have played an important role in the development of pesticides (Marrone, 2019). Federal registration to ensure set pesticide use and safety relies, in large part, on the ability to analyze the concentration of the active chemical with a high degree of sensitivity, accuracy, and precision. The pesticide analysis can be challenging for fermentation products, even when purified, when they are composed of multiple homologs that can vary greatly from batch-to-batch. High specificity using mass spectrometry is often required to effectively discriminate among homologs that vary only by functional group. Quantification by HPLC typically is performed by generating individual calibration curves for each homolog using appropriate chemical standards (Borrego and others, 1997; Bernardy and others 2013; de Souza and others, 2018). Here, we present an alternative approach by creating a single calibration curve using the averaged total response of the major ANT–A homologs (A1, A3, A3, A4) at each standard concentration (1.0–450.0 µg/L). The analysis is advantageous because it creates a more resilient ANT–A quantification method amendable to batch-batch differences in major homologs. Therefore, the present method demonstrates how ANT–A can be effectively measured in a liquid formulation with high accuracy (98–99 percent), precision, and specificity. The successful analytical method verification across three separate analyst groups demonstrated the method is reproducible and ready for consideration by regulatory applications.
Analytical chemistry methods continue to update with time, regulatory requirements, and the desire to develop timely, reliable, cost effective, and reproducible methods. Therefore, ANT–A analytical chemistry and sample processing techniques have continued to develop over the past several decades (Abidi, 1982; Bernardy and others, 2013). For example, mass spectrometry was first used as a qualitative identification of homologs by Abidi (1982), and other detectors were used for ANT–A quantification (for example, electrochemical, ultraviolet, fluorescence). Bernardy and others (2013) used mass spectrometry in single ion monitoring (SIM) mode to quantify ANT–A major homologs (A1–A4) and fragmentation products with high sensitivity. The present method utilizes mass spectrometry multiple reaction monitoring mode as opposed to SIM. This technique enables the sequential observation of major ANT–A homolog precursors, such as A1, A2, A3, and A4, simultaneously with their corresponding collision product ions (chemical fragments) to increase selectivity while maintaining comparable sensitivity. Earlier publications demonstrated ANT–A could be sufficiently resolved into major homologs by reversed-phase HPLC, and component separation was heavily dependent on mobile phase constituents, pH, and solvent composition (Abidi, 1982, 19882; Abidi and others, 1990). Bernardy and others (2013) similarly reported the ability to separate all homolog pairs but because each stereoisomeric pair of homologs were the same mass, the mobile phase gradient was adjusted to coelute each pair, increase method sensitivity, and decrease the number of peaks to integrate in the post-analysis. The present method utilized similar HPLC techniques previously reported but obtained sufficient major homolog separation with an advanced column and modified mobile phase solutions resulting in reduced retention times.
ANT–A is produced via Streptomyces sp. fermentation, and dissipation is affected by water content, pH, and temperature (Marking and Dawson, 1972). Recent analytical methodology and sample processing procedures (for example, anhydrous organic solvents, sodium sulfate) were developed to minimize chemical degradation and stabilize ANT–A for accurate quantification (Bernardy and others, 2013). New sample preparation replaced acetone for acetonitrile as the elution solvent and enhanced ANT–A stability and accuracy. The concentration of the active ingredient (ANT–A) in the solution 1 and 2 was sufficiently high. SPE cartridges were used to remove inert ingredients rather than “concentrate” sample concentration; therefore solution 1 and 2 were appropriately diluted for quantification within the standard calibration curve (~100 µg/L and 250 µg/L). Conversely, the Bernardy and others (2013) LC–MS method reported quantification limits low enough to be used for pesticide surface-water monitoring (~8 nanograms per liter). Wamboldt and others (2024) analytically verified ANT–A water concentrations in laboratory whole fish toxicity studies using the present described method to quantify ANT–A water concentrations <1 µg/L. The progression of analytical technology and methodology to quantify ANT–A has greatly improved over the past four decades (Abidi, 1982), especially considering previous methods to measure the presence and quality of ANT–A utilized biological activity in fish and yeast (Lee and others, 1971; Lennon and Vézina, 1973).
An analytical method was successfully verified to remove inert formulation ingredients and quantify total ANT–A using HPLC–QQQ. The verified method supports Federal pesticide registration requirements that aim to ensure safe pesticide use and product quality. The detection of structural analog precursor ions paired with product ions resulted in high accuracy, precision, and specificity to quantify ANT–A concentrations within the liquid formulation (solutions 1 and 2). Future ANT–A method development could determine if the present HPLC–QQQ methodology and previously reported water-sampling methods are capable of measuring an ANT–A liquid formulation in water during native fish population restoration efforts.
References Cited
Abidi, S.L., 1982, High-performance liquid chromatographic resolution and quantification of a dilactonic antibiotic mixture (antimycin A): Journal of Chromatography A, v. 234, no. 1, p. 187–200. [Also available at https://doi.org/10.1016/S0021-9673(00)81792-6.]
Abidi, S.L., 1988, High-performance liquid chromatographic separation of subcomponents of antimycin A: Journal of Chromatography A, v. 447, p. 65–79. [Also available at https://doi.org/10.1016/0021-9673(88)90007-6.]
Abidi, S.L., Ha, S.C., and Rosen, R.T., 1990, Liquid chromatography—Thermospray mass spectrometric study of N-acylamino dilactones and 4-butyrolactones derived from antimycin A: Journal of Chromatography A, v. 522, p. 179–194. [Also available at https://doi.org/10.1016/0021-9673(90)85188-2.]
Bernardy, J.A., Hubert, T.D., Ogorek, J.M., and Schmidt, L.J., 2013, Determination of antimycin-A in water by liquid chromatographic/mass spectrometry—Single-laboratory validation: Journal of AOAC International, v. 96, no. 2, p. 413–421. [Also available at https://doi.org/10.5740/jaoacint.12-286.]
Borrego, C.M., Garcia-Gil, L.J., Vila, X., Cristina, X.P., Figueras, J.B., and Abella, C.A., 1997, Distribution of bacteriochlorophyll homologs in natural populations of brown-colored phototrophic sulfur bacteria: FEMS Microbiology Ecology, v. 24, no. 4, p. 301–309. [Also available at https://doi.org/10.1111/j.1574-6941.1997.tb00447.x.]
de Souza, C.G., Martins, F.I.C.C., Zocolo, G.J., Figueiredo, J.E.F., Canuto, K.M., and de Brito, E.S., 2018, Simultaneous quantification of lipopeptide isoforms by UPLC-MS in the fermentation broth from Bacillus subtilis CNPMS22: Analytical and Bioanalytical Chemistry, v. 410, p. 6827–6836. [Also available at https://doi.org/10.1007/s00216-018-1281-6.]
Fredricks, K.T., Hubert, T.D., Amberg, J.J., Cupp, A.R., and Dawson, V.K., 2021, Chemical controls for an integrated pest management program: North American Journal of Fisheries Management, v. 41, no. 2, p. 289–300. [Also available at https://doi.org/10.1002/nafm.10339.]
Fuller, P.L., Nico, L.G., and Williams, J.D., 1999, Nonindigenous fishes introduced into the inland waters of the United States: Bethesda, Md., American Fisheries Society, Special Publication 27, 622 p. [Also available at https://doi.org/10.47886/9781888569148.]
Kolar, C.S., Courtenay, W.R., Jr., and Nico, L.G., 2010, Managing undesired and invading fishes, chap. 8 of Hubert, W.A., and Quist, M.C., eds., Inland fisheries management in North America (3d ed.): Bethesda, Md., American Fisheries Society, p. 213–259. [Also available at https://doi.org/10.47886/9781934874165.ch8.]
Lee, T.H., Derse, P.H., and Morton, S.D., 1971, Effects of physical and chemical conditions on the detoxification of antimycin: Transactions of the American Fisheries Society, v. 100, no. 1, p. 13–17. [Also available at https://doi.org/10.1577/1548-8659(1971)100<13:EOPACC>2.0.CO;2.]
Lennon, R.E., and Vézina, C., 1973, Antimycin A, a piscicidal antibiotic: Advances in Applied Microbiology, v. 16, p. 55–96. [Also available at https://doi.org/10.1016/S0065-2164(08)70023-6.]
Marking, L.L., and Dawson, V.K., 1972, The half-life of biological activity of antimycin determined by fish bioassay: Transactions of the American Fisheries Society, v. 101, no. 1, p. 100–105. [Also available at https://doi.org/10.1577/1548-8659(1972)101<100:THOBAO>2.0.CO;2.]
Marrone, P.G., 2019, Pesticidal natural products—Status and future potential: Pest Management Science, v. 75, no. 9, p. 2325–2340. [Also available at https://doi.org/10.1002/ps.5433.]
Saari, G.N., Steiner, J.N., Lada, B.M., and Carmosini, N., 2024, Data release for determination of Antimycin–A in liquid formulation by high performance liquid chromatography–mass spectrometry: U.S. Geological Survey data release, accessed November 2024 at https://doi.org/10.5066/P1GT55QY.
Tricarico, E., Junqueira, A.O.R., and Dudgeon, D., 2016, Alien species in aquatic environments—A selective comparison of coastal and inland waters in tropical and temperate latitudes: Aquatic Conservation—Marine and Freshwater Ecosystems, v. 26, no. 5, p. 872–891. [Also available at https://doi.org/10.1002/aqc.2711.]
Wamboldt, J.J., Steiner, J.N., Sauey, B.W., Lada, B.M., Putnam, J.G., Korducki, B.M., and Saari, G.N., 2024, Toxicity of a management bait for grass carp (Ctenopharyngodon idella) incorporated with antimycin A: Ecotoxicology, 12 p. [Also available at https://doi.org/10.1007/s10646-024-02771-x.]
Conversion Factors
Temperature in degrees Celsius (°C) may be converted to degrees Fahrenheit (°F) as follows:
°F = (1.8 × °C) + 32.
Temperature in degrees Fahrenheit (°F) may be converted to degrees Celsius (°C) as follows:
°C = (°F – 32) / 1.8.
Supplemental Information
Specific conductance is in microsiemens per centimeter at 25 degrees Celsius (µS/cm at 25 °C). Ultrapure water is purified water with resistivity at 18.2 megaohm (MΩ). Concentrations of chemical constituents in water are in either grams per liter (g/L), milligrams per liter (mg/L), micrograms per liter (µg/L), nanograms per liter (ng/L), or millimolar (mM), which refers to the unit of concentration molarity that is equal to the number of moles per liter of a solution. Liquid solution flow rates are in liters per minute (L/min) or milliliter per minute (mL/min). Volume of samples are in milliliters (mL). Particle sizes are in micrometers (µm).
Abbreviations
~
about
≥
greater than or equal to
<
less than
≤
less than or equal to
±
plus or minus
AA
ammonium acetate
ACN
acetonitrile
ACNFA
dried acetonitrile with 1-percent formic acid
AMH
ammonium hydroxide
ANT–A
antimycin–a
CAS
Chemical Abstracts Service
EPA
U.S. Environmental Protection Agency
FA
formic acid
HPLC
high performance liquid chromatography
LC–MS
liquid chromatography–mass spectrometry
MeOH
methanol
min
minute
QQQ
triple quadrupole mass spectrometer
r2
coefficient of determination
RSD
relative standard deviation
SE
standard error
SIM
single ion monitoring
UMESC
Upper Midwest Environmental Sciences Center
For more information concerning the research in this report, contact the
Director, Upper Midwest Environmental Sciences Center
U.S. Geological Survey
2630 Fanta Reed Road
La Crosse, Wisconsin 54603
Publishing support provided by the
Rolla and Sacramento Publishing Service Centers
Disclaimers
Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Although this information product, for the most part, is in the public domain, it also may contain copyrighted materials as noted in the text. Permission to reproduce copyrighted items must be secured from the copyright owner.
Suggested Citation
Saari, G.N., Steiner, J.N., Lada, B., and Carmosini, N., 2024, Determination of antimycin–a in a liquid formulation by high performance liquid chromatography–mass spectrometry: U.S. Geological Survey Open-File Report 2024–1068, 7 p., https://doi.org/10.3133/ofr20241068.
ISSN: 2331-1258 (online)
| Publication type | Report |
|---|---|
| Publication Subtype | USGS Numbered Series |
| Title | Determination of antimycin–a in a liquid formulation by high performance liquid chromatography–mass spectrometry |
| Series title | Open-File Report |
| Series number | 2024-1068 |
| DOI | 10.3133/ofr20241068 |
| Publication Date | November 18, 2024 |
| Year Published | 2024 |
| Language | English |
| Publisher | U.S. Geological Survey |
| Publisher location | Reston, VA |
| Contributing office(s) | Upper Midwest Environmental Sciences Center |
| Description | Report: vii, 7 p; Data Release |
| Online Only (Y/N) | Y |
| Additional Online Files (Y/N) | N |


