Anatidae Brood Records in Maine During Studies of Anas rubripes (American Black Duck), 1977–94
Links
- Document: Report (2.19 MB pdf) , HTML , XML
- Data Release: USGS data release - Waterfowl (Anatidae) Brood Data, Maine, 1977–1994
- Download citation as: RIS | Dublin Core
Preface
The studies described in this report were conducted by staff of the Maine Field Station of the Patuxent Wildlife Research Center while it was part of the U.S. Fish and Wildlife Service. The U.S. Fish and Wildlife Service documented a continuing decline in the Anas rubripes (Brewster, 1902) (American black duck) population and thus increased research efforts to determine the causes of the decline. While the studies described in this Data Report were conducted, the investigators and other field staff worked under the auspices of the U.S. Fish and Wildlife Service. In the fall of 1996, the programs and staff of Patuxent Wildlife Research Center, along with its Maine Field Station, were transferred to the U.S. Geological Survey.
The authors recognize the efforts of all the U.S. Fish and Wildlife Service bio-technicians who collected brood data for the studies discussed in this report. Authors especially thank James K. Ringelman who collected data on the Dixmont study site (1977–80) as a graduate student at the University of Maine. We also thank the many landowners who allowed access across their lands so we could reach wetlands to count broods.
Maurice Mills, Jr., (Moosehorn National Wildlife Refuge) provided insights about waterfowl and impoundment changes. R. Bradford Allen and Kelsey M. Sullivan (Maine Department of Inland Fisheries and Wildlife) provided agency reports on waterfowl status. Kelsey also reviewed a draft of this report. We thank them for their assistance.
William B. Krohn (U.S. Fish and Wildlife Service, Maine Cooperative Fish and Wildlife Research Unit) advised on use of data from the Gap Analysis of Maine. Guthrie S. Zimmerman (U.S. Fish and Wildlife Service, Population and Habitat Survey Branch) commented on the limitations and use of the Eastern Survey data. Mark R. Koneff (U.S. Fish and Wildlife Service, Branch of Migratory Bird Surveys) verified that wood duck and hooded merganser could not be reliably detected during the Eastern Survey. William J. Sheehan (Maine Department of Environmental Protection) commented on current use of Aroostook wetlands by duck species usually associated with prairie habitats.
Barbara S. Vickery, copyright holder of “Birds of Maine” by Peter D. Vickery and others (2020; published by Princeton University Press), granted us permission to use Map 3 (Land Use and Land Cover) on July 15, 2021. The generalized land cover classes shown in Map 3 were extracted from the Northeastern Terrestrial Habitat Classification System data of the Nature Conservancy, Eastern Conservation Science. William P. Hancock created Map 3 from these data and graciously modified it for this report by adding our study locations and the biophysical boundaries described by William B. Krohn and others in “Maine Gap Analysis: A Geographic Approach to Planning for Biological Diversity.” Joyce E. Longcore inserted locations of observation stands on the field map for figure 2. We thank these individuals for their assistance.
We thank Ducks Unlimited Canada’s Institute for Wetland and Waterfowl Research for providing financial and logistical support to the brood count data collection effort in Nova Scotia via helicopter and from elevated stands. We thank all the field biologists with Ducks Unlimited Canada for their professional collaboration.
The manuscript benefited from thoughtful reviews by John R. Sauer and Matthew C. Perry.
Abstract
This report describes a compilation of brood observations for Anatidae species breeding in Maine during an 18-year period (1977–94) that were made by the U.S. Geological Survey’s Patuxent Wildlife Research Center while it was operated by the U.S. Fish and Wildlife Service. During four focused studies, variables affecting the declining Anas rubripes (Brewster, 1902) (American black duck, hereafter black duck) population were assessed. Broods were observed on seven geographical study sites within four study areas located in three of Maine’s five biophysical regions. For combined studies, 168 wetlands were monitored for broods.
The 1,907 recorded broods were distributed among study areas: 185 in Dixmont, 241 in Cherryfield and Beddington, 411 in Moosehorn, Baring Unit and Edmunds Unit, and 849 in Aroostook County, Agricultural and Forested sites. Additionally, 221 broods were recorded in the 117-hectare Downing Bog wetland at the Cherryfield site during annual evening visits made between 1985 and 1991. Twelve Anatidae species, mostly black duck (676), Aix sponsa (Linnaeus, 1758) (wood duck; 265), Aythya collaris (Donovan, 1809) (ring-necked duck; 246), and Lophodytes cucullatus (Linnaeus, 1758) (hooded merganser; 163) were observed. Only 139 broods of Anas platyrhynchos (Linnaeus, 1758) (mallard) were found; all but 9 broods were at the Aroostook County, Agricultural site. Branta canadensis (Linnaeus, 1758) (Canada goose) broods were found at the Aroostook County, Agricultural site (58) and Moosehorn, Baring Unit (97).
For the combined studies, 468 of 676 (69.2 percent) of black duck broods reached fledging age (Class IIc-III), whereas 93 of 139 (66.9 percent) of mallard broods reached fledging age. Black ducks used predominantly palustrine, emergent wetland; palustrine, forested wetland; and palustrine, scrub-shrub wetland. Of the 134 mallard broods observed at the Agricultural and Forested sites in Aroostook County, 130 (97.0 percent) were observed at the Agricultural site and 93 (71.5 percent) of them were recorded on two palustrine and two lacustrine, unconsolidated bottom class wetlands. Mean size of black duck, wood duck, and ring-necked duck broods in this report were similar to those reported from historic Maine data.
Introduction
From the Atlantic seaboard to the upper Mississippi River and north into Canada lies a vast area of forested habitats and wetlands characterized by relatively stable water levels, Castor canadensis (Kuhl, 1820) (beaver)-created flowages, and bogs that produce large numbers of broods of waterfowl species each year. This approximately 1,036,000–1,295,000-square-kilometer (km2) habitat between the prairie-parklands and tundra, is an area known as the boreal forest and was not recognized as waterfowl habitat in the 1950s (Brazda, 1984). Although less fertile than the prairies, the boreal forest’s vastness and more stable wetlands produce ducks even when the prairies are affected during drought years. The open, closed, and mixed forests of the tundra and northern boreal forest area are perhaps more important to the continental waterfowl population than the prairies, although prairie habitats produce more ducks per km2 in wet years (Wellein and Lumsden, 1964).
Although Anas rubripes (Brewster, 1902) (American black duck, hereafter black duck) has historically bred in low numbers throughout this extensive landscape, its population had been declining since the mid-1950s (Rusch and others, 1989) as measured by the mid-winter index (Conroy and others, 1988). These findings stimulated Federal, State, and Provincial resource agencies to investigate causes of the decline. Although obtaining breeding population indices (pair-to-brood numbers, brood size, and brood densities) in the vast forested wetlands of the black duck’s range was an almost insurmountable inventory problem (Cowardin and Blohm, 1992), the agencies responsible acted because they deemed it essential to understand black duck population ecology to be able to set sustainable harvest management goals.
Four studies that investigated basic breeding ecology and potential causes of the black duck population decline in Maine were undertaken by Federal biologists between 1977 and 1994. Because of the commonality of brood observation methods, wetland classification, and water sampling, the studies are herein combined into a single release to provide historical context for comparison with future studies. The following Data Report and its associated data release present data on all Anatidae species, whereas previously published results focused on certain species (black duck or Aythya collaris [Donovan, 1809] [ring-necked duck]) or issues (effects of acid rain). Brood counts as well as data characterizing wetlands and visits to wetlands are available in the data release associated with this report (Longcore and Bunck, 2024).
Description of Study Areas
Anatidae brood data were collected from seven study sites in forested regions of Maine across four studies between 1977 and 1994 (fig. 1); each study had specific objectives (table 1). Study 1, which monitored years 1977–80, was conducted on the 151 km2 Dixmont site (DIXM) in the southern Dixmont and Newburgh townships and northern Jackson and Monroe townships of south-central Maine (lat. 44o38’53” N, long. 69o5’35” W1 ). Between 1955 and 1974, black duck numbers recorded in the mid-winter inventory of the Atlantic and Mississippi Flyways declined more than 40 percent (Bellrose, 1976). In 1976, the U.S. Fish and Wildlife Service (FWS) sought to determine which variables were contributing to this decline and assigned a biologist to initiate studies on ecology of breeding black ducks in Maine and a biologist to investigate aspects of wintering ecology in Maryland. The DIXM study site was chosen because historical wetland and waterfowl data were available from a Maine Department of Inland Fisheries and Wildlife study conducted from 1958 to 1960 (Spencer, 1963). The site’s geology is glacial till. Its topography consists of rolling hills and well-drained, shallow-to-deep soils that are more fertile than those of the other study sites (except Aroostook County). Much of the landscape was previously farmed land reverting to old-field succession but contains kettle-hole ponds and numerous beaver flowages associated with permanent streams (Ringelman, 1980). Longcore and Ringelman (1980) reported on variables that affected waterfowl breeding densities at DIXM. They also determined the survival rate (Ringelman and Longcore, 1982a), movements of, and habitat selection by juvenile black ducks (Ringelman and Longcore, 1982b).
Coordinates listed in this report locate the approximate center of each study site.
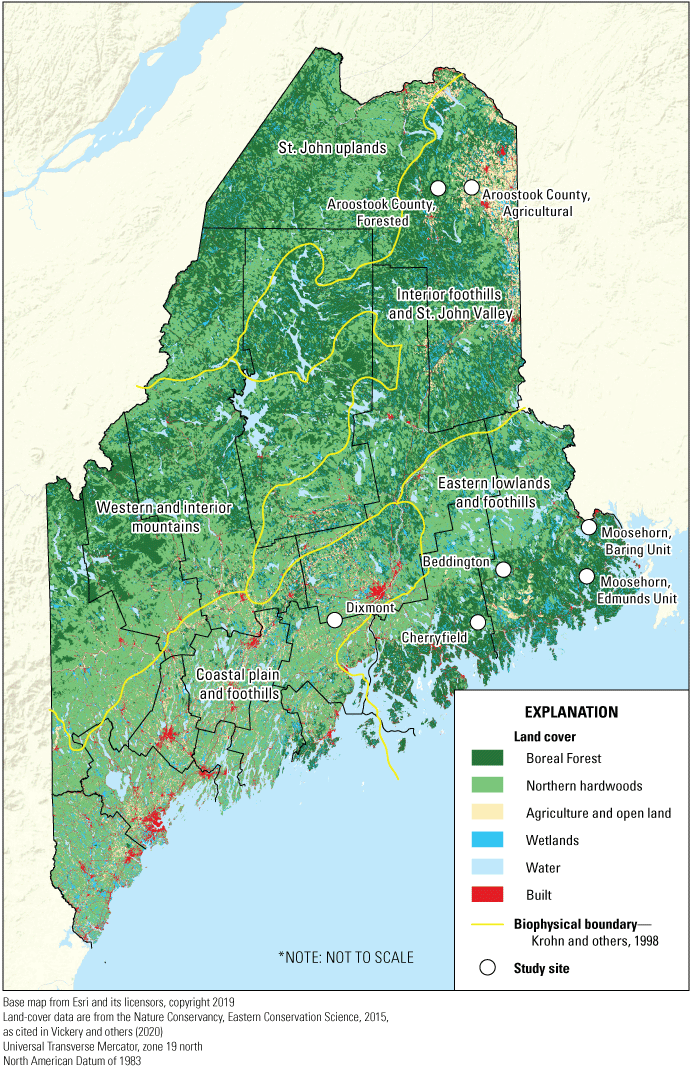
Map of Maine showing seven sites studied between 1977 and 1994 in relation to five biophysical regions and land-use type.
Table 1.
Background information for ecological studies of Anas rubripes (Brewster, 1902) (American black duck) and Anas platyrhynchos (Linnaeus, 1758) (mallard) in Maine, 1977–94.[Class IIc-III, duckling plumage class, 34–44 days (Gollop and Marshall, 1954); ANC, acid-neutralizing capacity; FWS, U.S. Fish and Wildlife Service; biophysical region, land use type (Krohn and others, 1998); km2, square kilometer]
Study 2, which monitored years 1982–84, included two sites, the 66 km2 Cherryfield site (CHER) located mostly in Township 10 SD (lat. 44o38’14” N, long. 68o04’26” W) and the 25 km2 Beddington site (BEDD) located in Township 30 MD (lat. 44o54’30” N, long. 67o52’51” W) in southeastern Maine (fig. 1). These sites were chosen because the region had substrates with low acid-neutralizing capacity (ANC) that have low specific conductivity and low pH (Norton, 1980). Both variables may affect the productivity of wetlands and thereby survival of ducklings by limiting invertebrate foods (Blancher and McAuley, 1987; Staicer and others, 1994). Surficial geology at these sites is a mix of glacial till, granitic bedrock, and soils that vary from thin loams to sandy and gravelly soils (Thompson and Borns, 1985). Most habitats in these areas had never been farmed and were managed for forest products (Barton and others, 2013). Longcore and others (2006) evaluated physical and chemical characteristics of wetlands in these two study sites and analyzed how macroinvertebrate abundance (on 10 sampled wetlands) and wetland characteristics affected wetland use by avian species, including Anatidae species. Survival of juvenile ring-necked ducks, their foods, along with nesting phenology, were reported by McAuley and Longcore (1988a, b; 1989). Woodcock and others (2005) evaluated the role of pH and macrophytes in affecting chironomid larvae distribution in these 10 wetlands sampled for macroinvertebrates. After the end of Study 2, brood observations continued from 1985 to 1991 at a 117-hectare (ha) bog wetland enhanced by beaver. This unique and highly productive wetland, Downing Bog, was in the CHER site.
Study 3, which monitored site MOBU during 1983–85 and site MOEU during 1984–85, focused on these sites in the 116 km2 Moosehorn National Wildlife Refuge in Calais, Maine. The first site was the 81 km2 Moosehorn, Baring Unit (MOBU; lat. 45o02’00” N, long. 67o17’58” W; fig. 1). The second site was the 35 km2 Moosehorn, Edmunds Unit (MOEU; lat. 44o50’37” N, long. 67o12’34” W; fig. 1). Study 3 was undertaken at the request of the FWS, Regional Office, Boston, to assess production of the black duck in the refuge and to evaluate contributions made to their production by wetlands created by impounding water. A few existing natural wetlands adjacent to impoundments were also visited to detect broods. The geology of Moosehorn National Wildlife Refuge is characterized by granitic bedrock and thin, loamy soils. The refuge is mainly forested and contains natural ponds and 52 impounded wetlands, most of which were constructed in the 1930s; many are modified by beaver (Hierl and others, 2007). Because some impoundments were dewatered for structural repairs for some of the monitored years, not all impounded wetlands were available for study. Longcore and others (1987) reported on the occurrence of Anas platyrhynchos (Linnaeus, 1758) (mallard) in all study sites (except Aroostook County). Hierl and others (2007) used a multivariate adaptive approach to evaluate changes in 49 wetlands in the refuge mapped during years 1984–85 to compare to the same wetlands remapped in 2002. Suitability of the remapped wetlands for avian species was determined.
Study 4 took place in Aroostook County from 1993 to 1994 because both black ducks and mallards were found there in sufficient numbers for comparison of brood sizes on agricultural and forested wetlands. Aroostook County (lat. 46o47’47” N, long. 68o05’25” W) is an area of 3,462 km2 in northern Maine, adjacent to the state’s border with New Brunswick, Canada. Because of its size, the study focused on wetlands in the county’s central core, an area of 1,510 km2 called Aroostook County, Agricultural (AAGR), and the surrounding 1,952 km2, called Aroostook County, Forested (AFOR; fig. 1). Substrates in Aroostook County contained high ANC, which contrasted with the low ANC substrates of sites in Study 2. Wetlands in this landscape are underlain with glaciolacustrine sediments that support cedar swamps and peat lands with diverse forest ecosystems, including boreal forests (McMahon, 1990). The AAGR site is highly buffered, and soils are fertile (Norton, 1980). Numbers and sizes of black duck and mallard broods from this study were published by Longcore and others (1998).
Methods
Methods described in this section apply to all studies except as noted. Broods were considered the sampling unit on all study sites. All wetlands with suitable brood habitat were visited and monitored unless inaccessible (woods road impassible, culvert out). Some small wetlands and those without vegetative cover for concealment were excluded from monitoring after initial reconnaissance. The value of these data lies in the historical context of the ongoing warming climate and the associated emergence of weather events (extreme rainfall and temperatures; Huber and Gulledge, 2011) that may cause flooding or drought that affect waterfowl habitats.
Measuring Wetland Attributes and Characteristics
The wetlands were mapped with aerial photography. The resulting 10-by-10-inch black-and-white contact prints were ground proofed to delineate basin perimeter, vegetation, and shoreline, and then classified with the system of Cowardin and others (1979). Figure 2 is an example of a field map generated using this method. It depicts Downing Bog, a wetland in the CHER site that was monitored during Study 2 and an additional 7 years after the study’s completion. The system and class of the wetlands were designated according to the dominant (30-percent areal coverage or greater) form of a vegetative life. The maps of all monitored wetlands were updated annually except for those in Aroostook County, where the wetlands monitored were mapped only in 1994. The wetlands were grouped into nine classes based on predominant vegetation type:
-
• lacustrine, aquatic bed (LAB),
-
• lacustrine, rock bottom (LRB),
-
• lacustrine, unconsolidated bottom (LUB),
-
• palustrine, aquatic bed (PAB),
-
• palustrine, emergent wetland (PEW),
-
• palustrine, forested wetland (PFW),
-
• palustrine, rock bottom (PRB),
-
• palustrine, scrub-shrub (PSS), and
-
• palustrine, unconsolidated bottom (PUB).
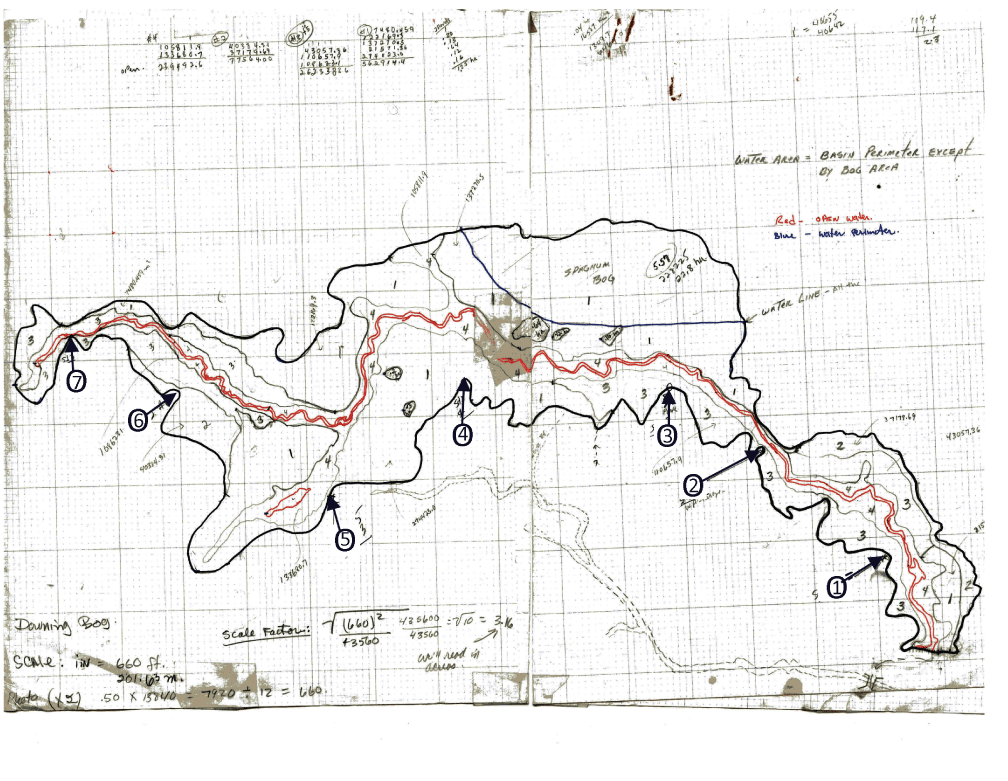
Example of a field map prepared using aerial photography and ground proofing methods for a wetland survey of Downing Bog, Cherryfield site, Maine. The seven observation stands used at Downing Bog during this period are indicated by bolded, circled numbers (1–7). Vegetative cover types are defined by numbers 1–4.
For surface water samples collected in July, specific conductance (mean μS/cm), an overall measure of nutrient content, and pH were reported for all wetlands except as noted in the table 2. These data were reported in 1979 for DIXM, 1982 for BEDD and CHER, 1984 for MOBU and MOEU, and 1994 for AAGR and AFOR. Samples from the midpoint of each wetland’s outlet were collected at 20–35 centimeters below the surface. In all sites except those in Aroostook County, specific conductance was measured with a Markson Science Inc. (Del Mar, CA), Model-10 meter; and pH, with a Fisher Scientific Company (Pittsburgh, PA), Model 640 portable, calibrated meter. In Aroostook County, samples were collected in containers rinsed in deionized water and transported on ice to the Environmental Chemistry Laboratory, Department of Geological Sciences, University of Maine–Orono for analysis. Detailed analytical procedures for water chemistry analyses for CHER and BEDD are in Longcore and others (2006), and those for AAGR and AFOR are in Longcore and others (1998).
Table 2.
Summary statistics for wetland size, conductance, and pH by study and site in Maine, 1977–94.[Data are from Longcore and Bunck (2024). N, number of wetlands; ha, hectare; µS/cm, microsiemens per centimeter; DIXM, Dixmont; BEDD, Beddington; CHER, Cherryfield; MOBU, Moosehorn, Baring Unit; MOEU, Moosehorn, Edmunds Unit; AAGR, Aroostook County, Agricultural; AFOR, Aroostook County, Forested]
Protocol for Observing and Recording Broods
Each brood-rearing wetland was visited at least eight times per 7–10-day period to ensure black duck broods could be followed from hatching (Class Ia) to fledging (Class IIc-III; 34–44 days; Gollop and Marshall, 1954). Brood use, including all Anatidae species, was determined by observing wetlands for 2 hours each visit from May to early September. Observations began 0.5 hour before sunrise or ended 0.5 hour after sunset from elevated platforms (Longcore and Ringelman, 1980). The species of a brood was identified by observing the accompanying female along with the physical characteristics and plumage of ducklings; color images of downy young in Kortright (1942) were useful to confirm species. The number and age class of ducklings and their species was used to identify individual broods. Many wetlands were visited a total of 10–11 times to document the fledging date of a brood (table 3). For most wetlands, a single observer was adequate for a count; however, as many as 6 or 7 observers were needed to cover large wetlands such as the highly productive Downing Bog (fig. 2).
Table 3.
Number of visits per wetland by study, site, and year in Maine, 1977–94.[Data are from Longcore and Bunck (2024). DIXM, Dixmont; BEDD, Beddington; CHER, Cherryfield; MOBU, Moosehorn, Baring Unit; MOEU, Moosehorn, Edmunds Unit; AAGR, Aroostook County, Agricultural; AFOR, Aroostook County, Forested]
Timing and Duration of Field Seasons
The onset and length of breeding periods can vary among species and years depending on environmental variables, especially weather that affects breeding ground habitats such as ice-out. Breeding pairs of black ducks usually arrive on Maine wetlands in early April and commence egg laying by mid-April; nests started later than May 10 are considered renests (Coulter and Miller, 1968). The timing of the initial visit to each wetland differed among study sites and years but were reasonably consistent overall (table 4). Visits starting in late May at the DIXM site reflected the team’s unfamiliarity with the habitat, especially in the first year, 1977. More time was spent looking at wetlands without broods because not all the wetlands were evaluated for their suitability for broods. Also, fewer than 10 elevated observation platforms were erected in 1977 (Longcore and Ringelman, 1980). The initial date of wetland visits in 1993 was identical for AAGR and AFOR sites but was about one week later in 1994 for the AFOR site related to logistics of locating suitable, scattered wetlands in the more remote, forested habitats.
Table 4.
Timing and duration of wetland visits, by study, site, and year in Maine, 1977–94.[Data are from Longcore and Bunck (2024). DIXM, Dixmont; BEDD, Beddington; CHER, Cherryfield; MOBU, Moosehorn, Baring Unit; MOEU, Moosehorn, Edmunds Unit; AAGR, Aroostook County, Agricultural; AFOR, Aroostook County, Forested]
Aging Techniques for Ducklings and Goslings
The ages of ducklings were estimated by evaluating their shape and size, pattern of down replacement, and degree of feather emergence (Kortright, 1942; Gollop and Marshall, 1954), characteristics that Evrard (1996) evaluated and found reliable. The generalized duckling plumage classes and sub-classes of Gollop and Marshall (1954) are as follows: Ia, a bright ball of fluff; Ib, a fading ball of fluff; lc, gawky-downy; IIa, first feathers; llb, mostly feathered; llc, last down; and III, feathered-flightless. Refer to Gollop and Marshall (1954) for additional descriptors. Images of developing Branta canadensis (Linnaeus, 1758) (Canada goose) goslings by Yocom and Harris (1965) depicted plumage classes, following the same age classes designated by Gollop and Marshall (1954), which were used to determine relative age. Grice and Rogers (1965) in Massachusetts published descriptions of wood duck duckling plumages in different age classes that were used to confirm age classes of ducklings we observed.
Protocol to Avoid Double Counting Broods
Flow charts were constructed for each brood on each wetland for each count to track the development of individual broods. After counts, the species, age, and number of ducklings in each brood were compared, noting the time of observation and the direction of brood movements to avoid double counting broods. The plumage development of a previously seen brood was then projected forward to the next observation period to seek a match for age and number of young. This was done with a two-part “slide rule.” The first part used a linear calendar that covered the period from the date the earliest brood hatched through the date the latest brood attained flight (Gollop and Marshall, 1954). The second part was a series of sliding strips, one for each species, that depicted the width (in days) of the seven age classes (Gollop and Marshall, 1954, app. 1). If no obvious match was found, a brood of the expected age, but with fewer ducklings, was sought. Broods were given a ±4-day leeway to limit the possibility of misclassifying a brood as “not seen previously” when it had been seen before. If initial aging of a brood was uncertain, an additional leeway of ±1 age class was allowed. This methodology may slightly underestimate the total number of broods, especially on wetlands with large numbers of broods of the same species, but ensures reliable, conservative estimates of brood size.
Results
This section describes the number of wetlands monitored, two key wetland water chemistry variables (pH and specific conductance), types of vegetation as classified by the Cowardin system (Cowardin and others, 1979), numbers of Anatidae broods observed by study area, and percentage of broods that reached fledging (Class IIc–III). Data are from Longcore and Bunck (2024).
General Characteristics of Wetlands
The size of a wetland, its amount and distribution of vegetative classes, and its water chemistry, especially acidity and specific conductance, which reflects nutrient content, affect which wetlands are used by brood-rearing Anatidae species.
Number of Wetlands
Between 1977 and 1994, broods were recorded on 145 of 168 monitored wetlands (table 2). The numbers of wetlands monitored during Studies 1, 2, and 3 were comparable (table 2). In these studies, all wetlands were evaluated to assess their suitability to support broods. Wetlands in Aroostook County were too numerous and often difficult to reach, thus not all wetlands were evaluated. Some wetlands in the AAGR site among crops were off limits because farmers wanted to avoid the possibility of plant diseases being brought in by the field crew. For all four studies, sites with wetlands of sufficient size and with suitable brood-rearing habitat were monitored, including all wetlands in which a brood was observed during an evaluation visit.
Size of Wetlands
The average size of the 168 monitored wetlands, as classified by the system established by Cowardin and others (1979), was smaller than 10.6 ha. Among the four studies, means were similar (Study 1, site mean was 6.5 ha; Study 2, site means were 5.9–13.4 ha; Study 3, site means were 4.0–12.8 ha; Study 4, site means were 8.3–14.0 ha; table 2). Approximately 85 percent of the wetlands for all studies did not exceed 13 ha (fig. 3); however, wetlands 20 ha or larger were in all study sites except BEDD and MOEU. In Study 1, the combined upper and lower Chase Bog was the largest wetland (82 ha). Study 2 monitored one large wetland, Downing Bog (117 ha); whereas the 143-ha Magurrewock complex, which was dissected by earthen dikes that created several compartments at MOBU, was the largest wetland in Study 3. In Study 4, nine large lakes (34–109 ha) and Christina Reservoir (113 ha) were monitored.
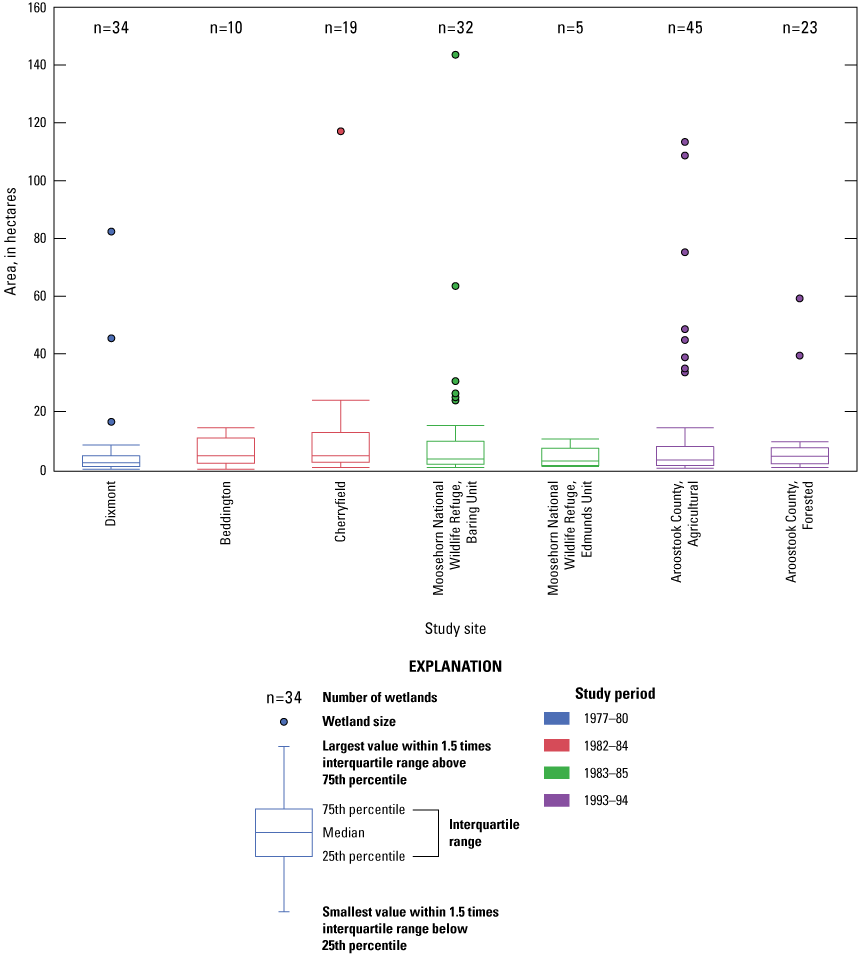
Boxplots showing the distribution of wetland size per study site in Maine monitored between 1977 and 1994. Data are from Longcore and Bunck (2024).
Wetland Acidity
Mean pH and range for wetlands in Study 1, Study 3, and one site in Study 4 (AFOR) were nearly circumneutral (table 2; fig. 4). Wetlands in the CHER and BEDD sites were the most acidic with a mean pH of 5.8, which is expected because the sites were selected to evaluate effects of acid rain on black duck production (Longcore and others, 2006). The agricultural and prairie-like landscape of the AAGR site had wetlands with higher mean pH (7.7) than other sites (table 2; fig. 4).
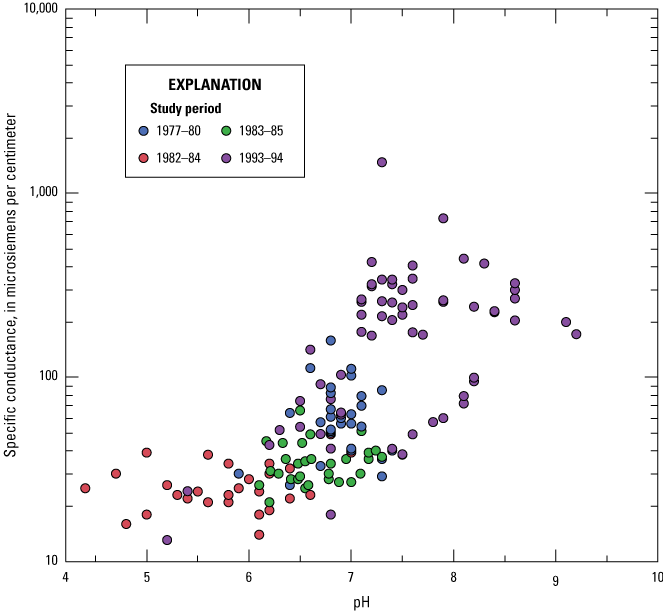
Plot comparing the pH and specific conductance of wetlands in Maine monitored between 1977 and 1994. Data are from Longcore and Bunck (2024).
Specific Conductance
The specific conductance of water in wetlands was lowest in Study 2; the CHER and BEDD sites had a mean µS/cm of 26 and 28, respectively. The two sites were in a landscape containing bedrock with low ANC. The highest conductance was measured during Study 4; the AAGR site, in which some wetlands were affected by nutrients from a food processing plant (Longcore and others, 1998) and had a mean µS/cm of 294 (table 2; fig. 4). The mean conductance of wetlands in Study 1 (DIXM; 63 µS/cm) and those in the AFOR site in Study 4 (65 µS/cm) were moderate. The mean conductance of wetlands in Study 3 (MOBU, 33 µS/cm; MOEU, 57 µS/cm [based on two wetlands]) was intermediate. The relationship between conductance and pH is one of values increasing together (fig. 4).
Wetland Classes Following the Cowardin Classification System
The 168 wetlands monitored during the four studies accounted for 1,788 ha. In Study 1 (DIXM), 223 ha were monitored; in Study 2 (BEDD, CHER), 313 ha; in Study 3 (MOBU, MOEU), 429 ha; and in Study 4 (AAGR, AFOR), 823 ha (fig. 5). The classes of wetlands differed substantially among the study sites. The wetlands in the mixed forested and farmland landscape of DIXM were mainly classified as PFW (42.0 percent), PSS (36.1 percent), and PEW (15.7 percent). In Study 2, the BEDD wetlands were mostly lacustrine (44.3 percent LAB, LRB, or LUB) and PSS (41.6 percent). The CHER site was similar, consisting mostly of lacustrine (24.6 percent LAB, LRB, or LUB), PSS (54.1 percent), and PAB (12.2 percent) wetlands. Study 3 wetlands differed greatly from the others. Those in MOBU were mainly classified as PEW (77.3 percent) and lacustrine (15.6 percent LAB LRB, or LUB). All wetlands in MOEU were classified as either PEW (73.1 percent) or PAB (26.9 percent). In Study 4, the wetlands in AAGR were mainly lacustrine (36.3 percent LAB, LRB, or LUB), PUB (26.0 percent), or PFW (26.4 percent). In contrast, the AFOR site was like the DIXM site, containing wetlands classified as PEW (36.9 percent), PFW (17.1 percent), and PSS (40.3 percent).
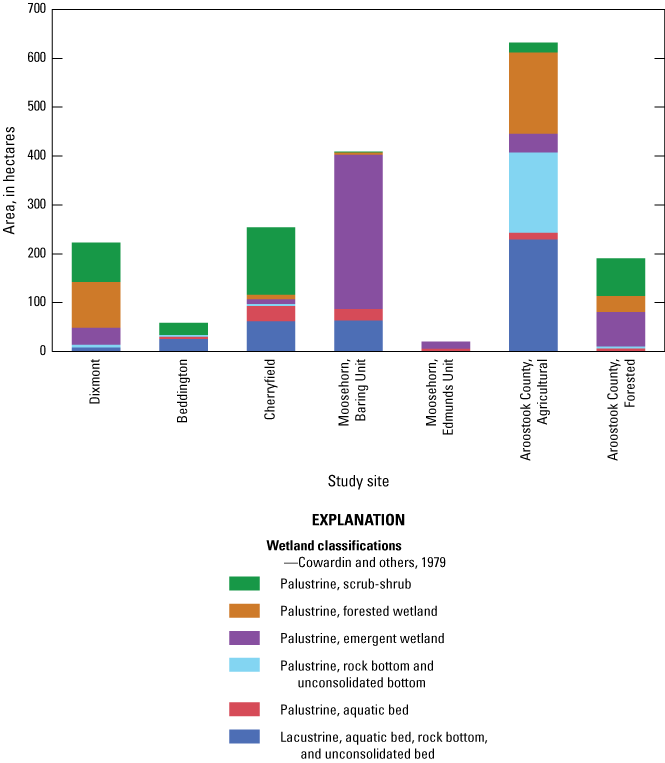
Bar graph showing the total hectares of seven study sites and the classifications of wetlands therein for four studies in Maine, 1977–94. Data are from Longcore and Bunck (2024).
Number of Visits and Observers per Visit
The mean number of visits to wetlands was variable among studies, partly because numbers of wetlands were different among sites and years (table 2). The lowest mean number of visits was during the initial field work (1977, DIXM) when field crews were unfamiliar with the wetland habitats. The number of wetlands increased during the 4-year study at the DIXM site because of beaver activity (Ringelman, 1980).
Anatidae Species Observed at Study Sites
Eleven duck species and one goose species were observed in the study sites (table 5). Black duck, ring-necked duck, and Lophodytes cucullatus (Linnaeus, 1758) (hooded merganser) raised broods on wetlands monitored in all four studies. Aix sponsa (Linnaeus, 1758) (wood duck) used all study sites but MOEU, and Anas crecca (Linnaeus, 1758) (green-winged teal) used all but BEDD. Mareca americana (J.F. Gmelin, 1789) (American wigeon) and Spatula clypeata (Linnaeus, 1758) (northern shoveler) used only AAGR. Only one or two mallard broods used the DIXM, BEDD, and CHER sites, whereas many were concentrated on the AAGR site. A few Spatula discors (Linnaeus, 1766) (blue-winged teal) broods used the CHER and MOBU sites, but most were counted in Study 4. A single Mergus merganser (Linnaeus, 1758) (common merganser) brood was observed in each of the DIXM, MOBU, and AFOR sites; four were counted on the CHER site. Bucephala clangula (Linnaeus, 1758) (common goldeneye) was observed only at BEDD and in Study 4. Canada geese raised broods only at the MOBU site and at both sites in Study 4 (AAGR and AFOR). Of the 1,907 broods observed on all sites, the black duck made up 35.4 percent, whereas wood duck made up 13.9 percent; ring-necked duck, 12.9 percent; hooded merganser, 8.5 percent; mallard, 7.3 percent; green-winged teal, 6.1 percent; and blue-winged teal, 3.7 percent (table 5). Each of the other four duck species made up less than 1.0 percent, or fewer than 20 broods. The 159 Canada goose broods made up 8.3 percent of the total broods.
Table 5.
Total number of observed broods by species, study, and site in Maine, 1977–94.[Data are from Longcore and Bunck (2024). ABDU, American black duck; AGWT, green-winged teal; BWTE, blue-winged teal; UNTE, unidentified teal; WODU, wood duck; MALL, mallard; AMWI, American wigeon; NSHO, northern shoveler; RNDU, ring-necked duck; HOME, hooded merganser; COGO, common goldeneye; COME, common merganser; CAGO, Canada goose; UNDU, unidentified duck; DIXM, Dixmont; BEDD, Beddington; CHER, Cherryfield; DOWN, Downing Bog; MOBU, Moosehorn, Baring Unit; MOEU, Moosehorn, Edmunds Unit; AAGR, Aroostook County, Agricultural; AFOR, Aroostook County, Forested]
Percentage of Broods by Species That Reached Class IIc–III (Fledging)
Of the black duck and mallard broods monitored during the four studies, 69.2 percent and 66.9 percent, respectively, were followed to fledging. These are the highest percentages among Anatidae species monitored because black duck and mallard were the focus of these studies (table 6). The highly visible Canada goose, with 56.6 percent of broods followed to fledging, was next highest. Final counts were more difficult to obtain for ducklings of other species, which tend to scatter (wood duck; McGilvrey, 1969) or dive (hooded merganser, common goldeneye). For these three species, the percent of broods with final counts ranged between 47.8 percent (hooded merganser) and 35.7 percent (common goldeneye). Because the ring-necked duck initiates nesting later, only 21.5 percent of its broods reached Class IIc-III before each field season ended (table 6).
Table 6.
Percentage of observed broods that reached fledging (Class IIc-III) by species in Maine, 1977–94.[Data are from Longcore and Bunck (2024). Data from all study sites and years are combined, including broods from Downing Bog (1985–91). Shows only species that were observed to have 10 or more broods that reached Class IIc-III. ABDU, American black duck; AGWT, green-winged teal; BWTE, blue-winged teal; MALL, mallard; WODU, wood duck; HOME, hooded merganser; RNDU, ring-necked duck; CAGO, Canada goose]
Discussion
Conditions necessary to obtain reliable data about the distribution and density of Anatidae species annually, or over multiple years, are confounded among many variables through time. Wetland habitats are diverse in configuration and the vegetational components may change often related to rainfall and the dynamic activities of beaver. Methods used for a brood survey, the seasonal timing of a survey, and the timing and duration of daily observations must be considered along with breeding patterns and behaviors of the species sought. The following topics examine variables that can affect obtaining reliable results.
Considering Brood Detectability
Detecting all broods of Anatidae species in boreal forest habitats of the Northeast with aerial or ground surveys is challenging. Some species are more readily detected (mallard, black duck, green-winged teal, ring-necked duck, and common goldeneye) than others. The wood duck and hooded merganser are poorly detected from fixed-wing aircraft. The three merganser species (hooded, common, and Mergus serrator [Linnaeus, 1758] [red-breasted]) are also not readily identified and are therefore lumped as “mergansers” during aerial surveys (Mark Koneff, Chief of the Branch of Migratory Bird Surveys, US Fish and Wildlife Service, oral commun., February 4, 2021).
Technicians participating in the ground surveys at the DIXM site in 1977 and the single-visit counts at Downing Bog in 1985–91 gained more understanding of the study area the longer the study area was observed. Pagano and Arnold (2009) found that novice observers on the ground during brood studies in prairie pothole habitats of North Dakota averaged 0.11 lower detection probabilities than more experienced observers.
Pagano and Arnold (2009) also reported that detection probabilities varied among duck species, time of observations (morning versus evening), wetland characteristics, and most critical, the duration of the survey (1 minute versus 5 minutes). Sauer (2002) cautions that survey methods must address two fundamental issues—an “accommodation of detectability” and identification of “sampling frames”—to ensure that all parts of the target population have a chance to be sampled. The 2-hour quiet observation survey either started 0.5 hour before sunrise or ended 0.5 hour after sunset, which ensured early and late-feeding broods could be detected. Whenever possible, intruding on a wetland was avoided. Multiple observers were deployed for counts to ensure wetland coverage. To visit wetlands during the entire brood period (early May to late September), 8 visits were made to each wetland every 7–10 days per study site (table 3). Counts were purposefully initiated in early May to observe Class I-aged broods; thus, age and size of broods did not affect counts as noted by others (Ringelman and Flake, 1980; Giudice, 2001).
Disturbance on or near a wetland causes females with ducklings to become secretive and sneak into the vegetation or even leave the wetland. Gabor and others (2000) found that sounds of an approaching helicopter will cause female dabbling ducks with broods to become motionless, leave a wetland, or swim under cover; hooded mergansers move to open water and dive. They recommended that quiet observation from elevated platforms be used to acquire reliable dabbling duck brood densities, duckling age, and number of ducklings. In 1995, some of the field crew in Nova Scotia verified their observational abilities by comparing counts made by a helicopter crew to that of a crew on elevated platforms (table 7). The helicopter crew missed 40–69 percent of the broods that were recorded by the crew on elevated platforms on five wetlands.
Table 7.
Number of duck broods determined by quiet observation on elevated platforms versus simultaneous counts by helicopter for selected wetlands in Nova Scotia, Canada, July 4–6, 1995.[ABDU, American black duck; NA, not applicable; AGWT, green-winged teal; WODU, wood duck; HOME, hooded merganser; —, no data; UNDU, unidentified duck; RNDU, ring-necked duck; MALL, mallard; BWTE, blue-winged teal. Data belong to Ducks Unlimited Canada. Published here with permission. Please contact Matt Dyson at Ducks Unlimited Canada for additional information.]
Changes Through Time for Brood Numbers
Waterfowl brood data from these four studies are reliable within a study site but attempting to determine a long-term trend for the 18-year period of Anatidae brood counts in Maine should be done with care. The occurrence and distribution of many wetlands are related to the activities of American beaver, so brood data are confounded over years, locations, and the changing conditions within study site wetlands. It is germane, however, to revisit any one of these study sites to evaluate changes over time. For example, the DIXM site would be ideal for determining the effect of land use, especially wetland dynamics, because of two previous studies by Spencer (1963) and Ringelman (1980) that published historical information for broods and wetlands for 1958–60 and 1977–80, respectively. For Study 2, the CHER site brood production was extraordinary (annually up to 1 brood per 2 ha of habitat) on Downing Bog. After the initial 3 years of the study, a single visit to count broods was made for an additional 7 years. Brood numbers declined during these 7 years; a future study to understand why may inform monitoring frequency, wetland changes, and the effect of a Haliaeetus leucocephalus (Linnaeus, 1766) (bald eagle) taking up residency. Data from Study 3 (MOBU and MOEU) is a robust baseline for determining the effects of changing impoundment management objectives, which could cause population changes in species (waterfowl, fish, amphibians). As for Study 4 (AAGR), environmental regulations applied to modify eutrophication in wastewater settling ponds (Christina Reservoir, Josephine Lake, nearby lagoons) may have already diminished waterfowl brood use since the study ended in 1994.
Each of the four studies contained 1–2 wetlands that accounted for a large proportion of total females with broods counted (table 8). For DIXM, Chase Bog (82 ha, class PFW) and Gilmore Meadows (5.7 ha, class PEW) accounted for 53.5 percent of the 185 broods counted during Study 1. For the CHER and BEDD sites, 56.0 percent of the 241 broods counted between 1982 and 1984 were on the Downing Bog (117 ha, class PSS). For the MOBU and MOEU sites, the Magurrewock complex (144 ha, class PEW) and Barn Meadow (64 ha, Class PEW) accounted for 37.2 percent of the 411 broods counted during Study 3. For the AAGR and AFOR sites, the Christina Reservoir (113 ha, class PUB) and Josephine Lake (45 ha, class PUB) wetlands accounted for 36.2 percent of the 849 broods counted during Study 4. These two wetlands were exceptionally rich in nutrients from the potato processing plant that maintained these settling basins (Longcore and others, 1998).
For some individual species, however, broods were not concentrated only on one wetland. For example, although 69.7 percent of black duck broods in Study 2 were on Downing Bog (CHER site), an additional 11.8 percent (9 broods) were on Snake Flowage (4.9 ha, class PFW). Longcore and McAuley (2004) counted four usual-sized broods (8–10 ducklings) and two large broods (one of 18–20 Class Ia-b ducklings and one of 20 Class Ib ducklings) in 1982. The size of the large broods likely resulted from a post-hatch amalgamation for two black duck broods. Thus, six black duck broods used Snake Flowage in 1982 along with three hooded merganser, two wood duck, and one green-winged teal brood. This wetland was a beaver flowage that had been dewatered several years before Study 2 began and then reflooded by beaver to create an excellent PFW habitat.
Table 8.
Examples of highly productive wetlands that supported a large proportion of broods for each study in Maine, 1977–94.[Data are from Longcore and Bunck (2024). ha, hectares; DIXM, Dixmont; PFW, palustrine, forested wetland; PEW, palustrine, emergent wetland; —, no data; CHER, Cherryfield; BEDD, Beddington; DOWN, Downing Bog; PSS, palustrine, scrub-shrub; MOBU, Moosehorn, Baring Unit; MOEU, Moosehorn, Edmunds Unit; AAGR, Aroostook County, Agricultural; AFOR, Aroostook County, Forested, PUB, palustrine, unconsolidated bottom]
Wetlands were classified using the system presented by Cowardin and others (1979).
Dispersal of an Introduced Species (Canada goose)
Introductions of the Canada goose established breeding populations in Maine. Palmer (1949) remarks that the Canada goose nested in Maine during the colonial period and cites several references from the 1800s into the mid-1900s. Although the Canada goose was recorded in Maine as early as 1605 (Rosier, 1887), the first authentic records of breeding in the state (nest of five eggs) was in 1939 in Penobscot County (Mendall, 1945). Then, in 1944, two broods were seen with adults in northern Piscataquis County near the Maine-Quebec border. These breeders were thought to be wild-strain birds, but the source could have been cripples from hunting, or escaped or feral birds.
The breeding geese at MOBU probably began from pinioned (18) and wing-clipped (16) birds released at Magurrewock marsh in 1952 (Moosehorn National Wildlife Refuge, 1952). In 1959, two Canada goose broods were documented at the refuge (Moosehorn National Wildlife Refuge, 1959), which constituted the first known goose nesting in eastern Maine since the 1940s. By 1965, the breeding population was well established; there were as many as 15 broods, based on young produced, and the pairs dispersed 20 miles from the refuge (Moosehorn National Wildlife Refuge, 1966). By 1990, the breeding population was stable with a minimum of 33 broods accounting for over 100 young annually (Moosehorn National Wildlife Refuge, 1991).
The Maine Department of Inland Fisheries and Wildlife initiated a Canada goose transplant project in 1965 by releasing 56–78 geese at each of three Wildlife Management Areas and 14 geese at MOEU (Spencer and Corr, 1976). Goose releases continued annually for 10 more years with a total of 2,341 geese from New York, New Jersey, and Connecticut being released mostly throughout southern Maine (Weik, 2005). During 1981–85, 1,723 more geese were moved from Connecticut into mostly northern Maine (Weik, 2005); some were released into Aroostook County townships where goose broods were counted in 1993–94. Canada geese are considered to be distributed statewide and broods are now associated with almost all release sites or adjoining areas (Vickery and others, 2020).
During Study 3, 97 Canada goose broods were recorded at MOBU, and during Study 4, 62 at AAGR and AFOR. The mean (± standard error [SE]) size of broods that reached Class IIc-III was 3.15±0.21 in MOBU (62 broods) and 3.79±0.30 in AAGR (28 broods), which may reflect the higher conductance of wetlands in the AAGR site that affected gosling survival. Mean (± SE) brood size with sites combined was 3.34±0.17 (90 broods). This value is lower than what Schummer and others (2011) reported (3.89±0.36; 28 broods) for the same years (1977–94; table 9). These data provide a reference point for comparison to future data that may describe distributional changes of Canada geese throughout Maine.
Table 9.
Historical sizes of waterfowl broods in Maine, 1955–2007 and 1977–94.[N, number of broods; SE, standard error; COME, common merganser; —, no data; HOME, hooded merganser; MALL, mallard; ABDU, American black duck; AMWI, American wigeon; AGWT, green-winged teal; BWTE, blue-winged teal; NSHO, northern shoveler; WODU, wood duck; RNDU, ring-necked duck; COGO, common goldeneye; CAGO, Canada goose]
| Species Group | Species alpha code1 | Maine Department of Inland Fisheries and Wildlife 2 | Longcore and Bunck (2024)3 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1955–2007 | 1977–944 | 1977–94 | ||||||||
| N | Mean | SE | N | Mean | SE | N | Mean | SE | ||
| Merganser | COME | 33 | 5.55 | 0.52 | 16 | 5.38 | 0.77 | 0 | — | — |
| HOME | 139 | 4.10 | 0.23 | 77 | 4.00 | 0.29 | 78 | 3.10 | 0.20 | |
| Dabbler | MALL | 55 | 3.96 | 0.30 | 31 | 4.00 | 0.39 | 93 | 4.61 | 0.30 |
| ABDU | 521 | 4.55 | 0.10 | 209 | 4.36 | 0.14 | 468 | 4.36 | 0.11 | |
| AMWI | 0 | — | — | 0 | — | — | 7 | 5.57 | 1.23 | |
| AGWT | 11 | 4.27 | 0.60 | 5 | 3.60 | 0.60 | 59 | 4.78 | 0.28 | |
| BWTE | 26 | 6.35 | 0.53 | 4 | 3.00 | — | 37 | 4.81 | 0.37 | |
| NSHO | 0 | — | — | 0 | — | — | 2 | 4.00 | — | |
| WODU | 206 | 3.63 | 0.15 | 96 | 3.81 | 0.22 | 97 | 3.67 | 0.18 | |
| Diver | RNDU | 69 | 4.46 | 0.31 | 21 | 4.48 | 0.46 | 53 | 4.34 | 0.29 |
| COGO | 179 | 4.14 | 0.19 | 95 | 4.36 | 0.28 | 5 | 3.40 | 1.12 | |
| Goose | CAGO | 75 | 4.15 | 0.25 | 28 | 3.89 | 0.36 | 90 | 3.34 | 0.17 |
Data were provided by Kelsey M. Sullivan (Maine Department of Inland Fisheries and Wildlife, unpub. data, 2023). At the time of publication, these data were not publicly available from the Maine Department of Inland Fisheries and Wildlife. Analysis of these data is publicly available from Schummer and others (2011). Data only includes Class III broods.
This data summary was constructed from a subset of the data collected by Schummer and others (2011) that corresponded to the same years monitored during the four studies summarized by Longcore and Bunck (2024).
Limitations on Interpreting Percent of Fledged Waterfowl
Nearly 69 percent of black duck broods and 66 percent of mallard broods reached fledging compared to 52 percent of blue-winged teal, 50 percent of green-winged teal, 47 percent of hooded mergansers, and 36 percent of wood ducks (table 6). Because black ducks and mallards were the major focus of the four studies discussed in this report, more effort was expended to obtain a final count of older broods, including returning for extra visits to wetlands with these species and timing the consecutive daily observations to coincide with the predicted date the brood reached Class IIc. When the observation period ended, some of the ring-necked duck broods had not reached Class IIc or III because the accompanying female had initiated nesting later, thus only 21 percent of the species’ broods were monitored to fledging. Difficulties with re-sighting broods on forested wetlands because tree canopies impair visibility, and the behavior of wood duck ducklings to scatter (McGilvrey, 1969) and hooded merganser and common goldeneye to dive (Bellrose, 1976) may have contributed to the lower percentages observed for these species.
Average Brood Sizes
During 1977–94 mean (±SE) brood sizes for black duck monitored to age Class IIc-III was 4.36±0.11 (468 broods); that of mallard, 4.61±0.30 (93 broods); that of wood duck, 3.67±0.18 (97 broods); that of hooded merganser 3.10±0.20 (78 broods); that of ring-necked duck, 4.34±0.29 (53 broods); that of green-winged teal, 4.78±0.28 (59 broods); and that of blue-winged teal, 4.81±0.33 (37 broods; table 9). In previous years and locations, average brood sizes were larger. Mendall (1958) reported a mean of 5.2 Class III ducklings per brood for 141 ring-necked duck broods during 1939–54 in Maine. Survival of 135 web-tagged wood duck broods in Massachusetts during 1952–53 averaged 5.8 ducklings per brood (Grice and Rogers, 1965). In Maryland during 1964–67, Class IIb aged wood duck broods averaged 5.2 for 223 broods (McGilvrey, 1969).
Schummer and others (2011) calculated the mean (±SE) Class III brood size for black duck (4.55±0.10), mallard (3.96±0.30), hooded merganser (4.10±0.23), common goldeneye (4.14±0.19), wood duck (3.63±0.15) and ring-necked duck (4.46±0.31) based on 51 years of counts in Maine during 1955–2007. During this period, 6–57 broods per year (23 on average) were counted, totaling 1,169 broods by 2007. No density values were presented for locally delineated wetland types (McCall, 1972) and no explanatory variable, including wetland type, received much support in their models. Survey year explained the most variability; however, only two counts were done per year per wetland, either by canoe or stationary ground blinds, and numbers of wetlands surveyed ranged from 20 to 63 per year. The actual effort Schummer and others (2011) expended per wetland per year, because of their acknowledged “cursory nature of our brood surveys,” likely affected results, but to what extent is unknown.
When the 1977–94 brood sizes reported by Schummer and others (2011) and Longcore and Bunck (2024) are compared (table 9), the mean (±SE) brood sizes are identical for black duck, are nearly identical for wood duck and ring-necked duck, but are 0.6 ducklings higher for mallard than for the Schummer and others (2011) data. The common goldeneye brood size (3.4±1.12) based on 5 broods averaged substantially lower than that of Schummer and others (2011; 4.36±0.28; 95 broods detected on 17 wetlands). The hooded merganser brood size from the Longcore and Bunck (2024) data was 0.9 ducklings fewer, but number of single-duckling Class III broods was about the same, 15 versus 13. The two sources of data were from different wetlands except for Arnold Brook Lake in Aroostook County, which Schummer and others (2011) monitored sporadically from 1985 to 1989.
The Canada goose mean (±SE) brood size with sites combined was 3.34±0.17 (90 broods). This value is lower than what Schummer and others (2011) reported (3.89±0.36; 28 broods) for the same years (1977–94; table 9).
It is prudent to remember that the average size of broods at fledging is subject to certain biases (Cowardin and Blohm, 1992); total brood loss and larger, early-hatched broods from larger clutches may affect ultimate size. The surveys of this report extended from May 1 to September 2 through the breeding season, which probably mitigated some effect except for loss of a total brood.
Special Case: Downing Bog, 10 Years of Visits
After the initial 3 years of brood evaluations, Downing Bog was monitored for broods (table 10) with one evening visit annually for an additional 7 years as part of Study 2, which evaluated effects of acid rain on conductance and pH on invertebrate diversity and numbers (Longcore and others, 2006). Downing Bog was of particular interest because of its low conductance (18 μS/cm) and pH (5.1). The wetland supported substantial numbers of black duck broods, counts of which provided an additional sample of final brood count (76) that was used to calculate mean size of Class IIc-III broods.
This single-visit effort revealed the benefit of multi-visit counts. The total number of Class IIc-III broods observed during the multi-visit counts in 1982–84 (90 of 135 total observed broods; 66.6 percent) was 22.7 greater than the total observed during single-visit counts in 1985–91 (97 of 221; 43.9 percent). Furthermore, a single visit may reflect unique annual habitat conditions from beaver activity or sparse rainfall and an aerial predator (like bald eagle) taking up residence near the wetland.
Table 10.
Number of waterfowl broods by species, date, and effort on the 117-hectare Downing Bog at the Cherryfield study site, Maine, 1982–91.[Data are from Longcore and Bunck (2024). ABDU, American black duck; MALL, mallard; AGWT, green-winged teal; BWTE, blue-winged teal; WODU, wood duck; HOME, hooded merganser; RNDU, ring-necked duck; BAEG, bald eagle; NA, not applicable]
Summary
A series of four studies from 1977 to 1994 that yielded data on 1,907 waterfowl broods was stimulated by the long-term decline of the Anas rubripes (Brewster, 1902) (American black duck, hereafter black duck) population. The decline was attributed to the Anas platyrhynchos (Linnaeus, 1758) (mallard), which was thought to negatively affect black duck productivity through hybridization or competitive exclusion from wetlands (Johnsgard and DiSilvestro, 1976). Studies were undertaken to investigate basic ecology of the black duck, determine effects of acid rain (using pH and specific conductance values) on invertebrates (Longcore and others, 2006), observe brood productivity on managed impoundments, and compare average brood sizes of black ducks and mallards in wetlands in agricultural and forested landscapes.
Field maps were prepared from aerial photographs for study wetlands; vegetation was classified following the system described by Cowardin and others (1979). Specific conductance and pH of water samples were measured for all but 13 wetlands. In total, 168 wetlands were monitored for Anatidae broods across seven study sites.
A standard protocol of quiet observation from elevated platforms, with 2-hour visits to wetlands beginning 0.5 hour before sunrise or ending 0.5 hour after sunset was followed. Each wetland was visited approximately eight times every 7 to 10 days. Visits began in May and extended into early September to cover the brood season. Flow charts of broods on each wetland were used to avoid double counting and follow the changes in size and number of ducklings or goslings. Use of a unique slide rule that depicted a calendar of the brood season that was meshed with insert strips of age classes of the species facilitated this effort.
The average size of the 168 monitored wetlands ranged from 4.0 to 14.0 hectares (ha) among the seven study sites: Dixmont (DIXM); Cherryfield (CHER); Beddington (BEDD); Moosehorn, Baring Unit (MOBU); Moosehorn, Edmunds Unit (MOEU); Aroostook County, Agricultural (AAGR); and Aroostook County, Forested (AFOR). A few wetlands were less than or equal to 1 ha; the largest was 143.6 ha. Specific conductance of water samples averaged less than or equal to 65 microsiemens per centimeter (µS/cm) at all sites except for the AAGR site (294 µS/cm) with a maximum of 1,462 µS/cm at Josephine Lake, which was a settling pond of a food processing plant. The lowest mean pH (5.8–5.85) and minimum pH (4.4–4.7) were at the BEDD and CHER sites. The highest mean pH (7.1–7.7) and maximum pH (8.4–9.2) were at the AAGR and AFOR sites.
For these combined studies, 468 of 676 (69.2 percent) of black duck broods reached fledging age (Class IIc-III), whereas 93 of 139 (66.9 percent) of mallard broods reached fledging age. Throughout the four study areas black ducks used the predominant wetlands: palustrine, emergent wetland; palustrine, forested wetland; and palustrine scrub-shrub wetland. In contrast, mallard broods (130 of 139, 93.5 percent) used predominately two palustrine and two lacustrine, unconsolidated bottom wetlands in Aroostook Country’s agricultural landscape.
Mean sizes of broods for three duck species (mallard, black duck, Aix sponsa [Linnaeus, 1758] [wood duck], and Aythya collaris [Donovan, 1809] [ring-necked duck]) from this report (Longcore and Bunck, 2024) were similar to those reported in an independent dataset (Kelsey M. Sullivan, Maine Department of Inland Fisheries and Wildlife, unpub. data, 2023) analyzed by Schummer and others (2011); although, different wetlands (except for Arnold Brook Lake) were surveyed by each source during 1977–94. Too few broods (10) were recorded for one or both surveys to calculate reliable means for six species: Mergus merganser (Linnaeus, 1758) (common merganser), Mareca americana (J. F. Gmelin, 1789) (American wigeon), Anas crecca (Linnaeus, 1758) (green-winged teal), Spatula discors (Linnaeus, 1766) (blue-winged teal), Spatula clypeata (Linnaeus, 1758) (northern shoveler), and Bucephala clangula (Linnaeus, 1758) (common goldeneye).
Broods of the following species mostly used the AAGR and AFOR site, which had high acid neutralizing capacities and included the nutrient-rich settling ponds: blue-winged teal (87.3 percent), mallard (96.4 percent), American wigeon (100 percent), northern shoveler (100 percent), and Branta canadensis (Linnaeus, 1758) (Canada goose; 39 percent). The highly productive wetlands of DIXM yielded 53.5 percent of the broods counted at the site; that of CHER/BEDD, 56.0 percent of broods counted; that of MOBU/MOEU, 37.2 percent; and that of AAGR/AFOR, 36.2 percent (table 8). These wetlands, excluding data from 7 years of single visits to Downing Bog, accounted for 41.2 percent of 1,686 broods recorded.
Methods chosen to meet the focused objectives of each study did not allow enough time to accommodate the later-nesting ring-necked duck. Only 21.5 percent of this species’ broods were observed to reach fledging. The establishment of nesting of Canada geese was aided by periodic introductions of adult breeding birds by the State of Maine. The species now breeds throughout Maine. Canada goose pairs fledged an average of 3.15 young at the MOBU site and 3.79 young at the AAGR site, the only sites where broods were observed. Data from these four studies may serve as useful historical markers for comparison with future waterfowl data collected while continuing effects of climate change alter weather patterns and subsequently wetland habitats.
References Cited
Barton, A., White, A., and Cogbill, C., 2013, Reconstructing the past—Maine forests then and now: Northern Woodlands, v. 77, summer issue, p. 40–47. [Also available at https://northernwoodlands.org/articles/article/reconstructing-past-maine-forests.]
Conroy, M.J., Goldsberry, J.R., Hines, J.E., and Stotts, D.B., 1988, Evaluation of aerial transect surveys for wintering American Black Ducks: The Journal of Wildlife Management, v. 52, no. 4, p. 694–703, https://doi.org/10.2307/3800933.
Cowardin, L.M., Carter, V., Golet, F.C., and LaRoe, E.T., 1979, Classification of wetlands and deepwater habitats of the United States: Washington, D.C., U.S. Fish and Wildlife Service, 131 p. [Also available at https://doi.org/10.5962/bhl.title.4108.]
Cowardin, L.M., and Blohm, R.J., 1992, Breeding population inventories and measures of recruitment, in Batt, D.J., Afton, A.D., Anderson, M.G., Ankney, C.D., Johnson, D.H., Kadlec, J.A., and Krapu, G.L., eds., Ecology and Management of Breeding Waterfowl: Minneapolis, University of Minnesota Press, p. 423–445.
Gabor, T.S., Longcore, J.R., Murkin, H.R., and Arnason, A.N., 2000, Comparison of helicopter and ground surveys of waterfowl broods in southern Ontario, in Obbard, M.E., and McLaughlin, C.R., eds., vol. 55 of Northeast wildlife—Transactions of the Northeast Section, the Wildlife Society: Peterborough, Ontario, Canada, Wildlife Society, p. 11–19. [Also available at https://digital.libraries.psu.edu/digital/collection/newildlife/id/5789/rec/1.]
Hierl, L.A., Loftin, C.S., Longcore, J.R., McAuley, D.G., and Urban, D.L., 2007, A Multivariate assessment of changes in wetland habitat for waterbirds at Moosehorn National Wildlife Refuge, Maine, USA: Wetlands, v. 27, no. 1, p. 141–152. [Also available at https://doi.org/10.1672/0277-5212(2007)27[141:AMAOCI]2.0.CO;2.]
Huber, D.G., and Gulledge, J., 2011, Extreme weather and climate change—Understanding the link and managing the risk: Arlington, Va., Center for Climate and Energy Solutions, 12 p. [Also available at https://www.c2es.org/publications/extreme-weather-and-climate-change.]
Johnsgard, P.A., and DiSilvestro, R., 1976, Seventy-five years of changes in mallard-black duck ratios in eastern North America: American Birds, v. 30, no. 5, p. 905–908. [Also available at https://sora.unm.edu/node/112576.]
Longcore, J.R., and Ringelman, J.K., 1980, Factors affecting waterfowl breeding density and productivity estimates in the northeast, in Healy, W.M., Carey, A.B., Healy, G.B., Horwitz, E., McDowell, R.D., and Soutiere, E.C., eds., Transactions of the Northeast Section of the Wildlife Society—37th Northwest Fish and Wildlife Conference, Ellenville, N.Y., April 27–30, 1980, p. 169–181. [Also available at https://digital.libraries.psu.edu/digital/collection/newildlife/id/6568/rec/2.]
Longcore, J.R., Corr, P.O., and McAuley, D.G., 1987, Black duck-mallard interactions on breeding areas in Maine, in Sayre, M.W., and Brzezinski, L.M., eds., Transactions of the Northeast Section of the Wildlife Society—44th Northwest Fish and Wildlife Conference, Boston, Mass., 1987, p. 16–31. [Also available at https://digital.libraries.psu.edu/digital/collection/newildlife/id/6876/rec/1.]
Longcore, J.R., Clugston, D.A., and McAuley, D.G., 1998, Brood sizes of sympatric American black ducks and mallards in Maine: The Journal of Wildlife Management, v. 62, no. 1, p. 142–151. [Also available at https://doi.org/10.2307/3802272.]
Longcore, J.R., and McAuley, D.G., 2004, Extraordinary size and survival of American black duck, Anas rubripes, broods: Canadian Field Naturalist, v. 118, no. 1, p. 129–131. [Also available at https://doi.org/10.22621/cfn.v118i1.897.]
Longcore, J.R., McAuley, D.G., Pendelton, G.W., Bennatti, C.R., Mingo, T.M., and Stromborg, K.L., 2006, Macroinvertebrate abundance, water chemistry, and wetland characteristics affect use of wetlands by avian species in Maine, in Hanson, A.R., and Kerekes, J.J., eds., Limnology and aquatic birds—Proceedings of the fourth conference working group on aquatic birds of Societas Internationalis Limnologiae (SIL), Sackville, New Brunswick, Canada, August 3–7, 2003: Hydrobiologia, v. 567, p. 143–167. [Also available at https://doi.org/10.1007/978-1-4020-5556-0_12.]
Longcore, J.R., and Bunck, C.M., 2024, Waterfowl (Anatidae) Brood Data, Maine, 1977–1994: U.S. Geological Survey data release, https://doi.org/10.5066/P13TOYWG.
McAuley, D.G., and Longcore, J.R., 1988a, Survival of juvenile ring-necked ducks on wetlands of different pH: The Journal of Wildlife Management, v. 52, no. 2, p. 169–176. [Also available at https://doi.org/10.2307/3801219.]
McAuley, D.G., and Longcore, J.R., 1988b, Foods of juvenile ring-necked ducks, relationship to wetland pH: The Journal of Wildlife Management, v. 52, no. 2, p. 177–185. [Also available at https://doi.org/10.2307/3801220.]
McGilvrey, F.B., 1969, Survival in wood duck broods: The Journal of Wildlife Management, v. 33, no. 1, p. 73–76. [Also available at https://doi.org/10.2307/3799651.]
Mendall, H.L., 1945, Canada geese nesting in Maine: The Auk, v. 62, no. 4, p. 640–641. [Also available at https://doi.org/10.2307/4079839.]
Moosehorn National Wildlife Refuge, 1952, Narrative report—May–August, 1952: Calais, Maine, Moosehorn Wildlife Refuge, [variously paged, 23 p.]. [First made publicly available by the U.S. Fish and Wildlife Service on September 17, 2014, at https://ecos.fws.gov/ServCat/Reference/Profile/32216.]
Moosehorn National Wildlife Refuge, 1959, Narrative report—May, June, July, August, 1959: Calais, Maine, Moosehorn Wildlife Refuge, [variously paged, 53 p.]. [First made publicly available by the U.S. Fish and Wildlife Service on September 17, 2014, at https://ecos.fws.gov/ServCat/Reference/Profile/32225.]
Moosehorn National Wildlife Refuge, 1966, Moosehorn Wildlife Refuge—1965, Narrative report: Calais, Maine, Moosehorn Wildlife Refuge, [variously paged, 117 p.]. [First made publicly available by the U.S. Fish and Wildlife Service on September 17, 2014, at https://ecos.fws.gov/ServCat/Reference/Profile/32234.]
Moosehorn National Wildlife Refuge, 1991, Annual Narrative Report—Calendar year 1990: Calais, Maine, Moosehorn Wildlife Refuge, [variously paged, 165 p.]. [First made publicly available by the U.S. Fish and Wildlife Service on February 15, 2015, at https://ecos.fws.gov/ServCat/Reference/Profile/42378.]
Norton, S.A., 1980, Geological factors controlling the sensitivity of aquatic ecosystems to acid precipitation, in Shriner, D.S., Richmond, C.R., and Lindberg, S.E., eds., Atmospheric sulfur deposition—Environmental impact and health effects—2d Life Science Symposium, Gatlinburg, Tenn., October 14–18, 1979 [Proceedings]: Ann Arbor, Mich., Ann Arbor Science Publishers Inc., p. 521–531.
Pagano, A.M., and Arnold, T.W., 2009, Estimating detection probabilities of waterfowl broods from ground-based surveys: The Journal of Wildlife Management, v. 73, no. 5, p. 686–694. [Also available at https://doi.org/10.2193/2007-524.]
Ringelman, J.K., and Flake, L.D., 1980, Diurnal visibility and activity of blue-winged teal and mallard broods: The Journal of Wildlife Management, v. 44, no. 4, p. 822–829. [Also available at https://doi.org/10.2307/3808310.]
Ringelman, J.K., and Longcore, J.R., 1982a, Movements and wetland selection by brood-rearing black ducks: The Journal of Wildlife Management, v. 46, no. 3, p. 615–621. [Also available at https://doi.org/10.2307/3808551.]
Ringelman, J.K., and Longcore, J.R., 1982b, Survival of juvenile black ducks during brood rearing: The Journal of Wildlife Management, v. 46, no. 3, p. 622–628. [Also available at https://doi.org/10.2307/3808552.]
Rusch, D.H., Ankney, C.D., Boyd, H., Longcore, J.R., Montalbano, F., III, Ringelman, J.K., and Stotts, V.D., 1989, Population ecology and harvest of the American black duck—A review: Wildlife Society Bulletin, v. 17, no. 4, p. 379–406. [Also available at https://www.jstor.org/stable/3782702.]
Sauer, J.R., 2002, Bird census techniques, second edition: The Condor, v. 104, no. 3, p. 698–701. [Also available at https://doi.org/10.1093/condor/104.3.698.]
Schummer, M.L., Allen, R.B., and Wang, G., 2011, Sizes and long-term trends of duck broods in Maine, 1955–2007: Northeastern Naturalist, v. 18, no. 1, p. 73–86. [Also available at https://doi.org/10.1656/045.018.0107.]
Staicer, C.A., Freedman, B., Srivastava, D., Dowd, N., Kilgar, J., Haydan, J., Payne, F., and Pollock, T., 1994, Use of lakes by black duck broods in relation to biological, chemical, and physical features: Hydrobiologia, v. 279/280, p. 185–199. [Also available at https://doi.org/10.1007/BF00027853.]
Thompson, W.B., and Borns, H.W., Jr., eds., 1985, Surficial geologic map of Maine: Capitol Heights, Md., Williams & Heintz Map Corporation, 1 sheet, scale 1:500,000. [Also available at https://digitalmaine.com/mgs_maps/15/#:~:text=Wall%20map%20showing%20the%20surficial%20geology%20of%20Maine.%20Includes%20sites.]
U.S. Geological Survey, [undated], Species table and recommended band sizes: U.S. Geological Survey web page, accessed July 15, 2024, at https://www.pwrc.usgs.gov/BBL/Bander_Portal/login/speclist.php.
Weik, A., 2005, Waterfowl assessment, Final: Bangor, Maine, Maine Department of Inland Fisheries and Wildlife, 267 p. [Also available at https://www.maine.gov/ifw/docs/species/birds/waterfowl/speciesassessment.pdf.]
Woodcock, T., Longcore, J., McAuley, D., Mingo, T., Bennatti, C.R., and Stromborg, K., 2005, The role of pH in structuring communities of Maine wetland macrophytes and chironomid larvae (Diptera): Wetlands, v. 25, no. 2, p. 306–316. [Also available at https://doi.org/10.1672/7.]
Yocom, C.F., and Harris, S.W., 1965, Plumage descriptions and age data for Canada goose goslings: The Journal of Wildlife Management, v. 29, no. 4, p. 874–877. [Also available at https://doi.org/10.2307/3798565.]
Conversion Factors
Supplemental Information
Specific conductance is in microsiemens per centimeter at 25 degrees Celsius (µS/cm at 25 °C).
For more information, contact
Director, Eastern Ecological Science Center,
11649 Leetown Road
Kearneysville, WV 25430
Or go to our website at https://www.usgs.gov/centers/eesc.
Published support provided by the Baltimore Publishing Service Center.
Disclaimers
Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Although this information product, for the most part, is in the public domain, it also may contain copyrighted materials as noted in the text. Permission to reproduce copyrighted items must be secured from the copyright owner.
Suggested Citation
Longcore, J.R., Bunck, C.M., McAuley, D.G., and Clugston, D.A., 2024, Anatidae brood records in Maine during studies of Anas rubripes (American black duck), 1977–94: U.S. Geological Survey Data Report 1200, 25 p., https://doi.org/10.3133/dr1200.
ISSN: 2771-9448 (online)
Study Area
| Publication type | Report |
|---|---|
| Publication Subtype | USGS Numbered Series |
| Title | Anatidae brood records in Maine during studies of Anas rubripes (American black duck), 1977–94 |
| Series title | Data Report |
| Series number | 1200 |
| DOI | 10.3133/dr1200 |
| Publication Date | November 22, 2024 |
| Year Published | 2024 |
| Language | English |
| Publisher | U.S. Geological Survey |
| Publisher location | Reston, VA |
| Contributing office(s) | National Wildlife Health Center, Eastern Ecological Science Center |
| Description | Report: vii, 25 p.; Data Release |
| Country | United States |
| State | Maine |
| Online Only (Y/N) | Y |
| Additional Online Files (Y/N) | N |


