Biodiversity Surveys of Wake Atoll—Featuring Field Guides for Plants, Arthropods, and Herpetofauna
Links
- Document: Report (50 MB pdf) , HTML , XML
- Appendixes:
- Appendix B (25 MB pdf) - Appendix B
- Appendix C (3.5 MB pdf) - Appendix C
- Appendix D (8.4 MB pdf) - Appendix D
- Download citation as: RIS | Dublin Core
Acknowledgments
Logistics, research coordination, and leadership were provided by Stacie Hathaway and Robert Fisher of the U.S. Geological Survey Western Ecological Research Center (USGS-WERC). We appreciate the administrative support provided by the USGS-WERC San Diego and Sacramento offices. Additional administrative support was provided by the U.S. Geological Survey’s Pacific Island Ecosystems Research Center and the Hawai‘i Cooperative Studies Unit at the University of Hawai‘i at Hilo (UHH-HCSU). We thank Pacific Air Forces Regional Support Center (PRSC) for supporting this project, particularly Kristen Rex and Joel Helm, who provided background and encouragement. Access, transport, and accommodations were provided by the U.S. Air Force (USAF) and facilitated by Kristen Rex. We thank Captain Robert Gibson (Detachment 1, PRSC) for providing atoll-wide access and general support; Chugach Federal Solutions Inc. teams, especially the mess hall, fire department, lodging, logistical, and additional staff members who provided for our nourishment, safety, accommodations, and general well-being; and all others who graciously allowed our surveys to take place in their work and personal spaces. We extend many thanks to John Gilardi, who was on Wake Atoll when we performed the survey and provided useful information regarding the natural resources, locations of plant species, and ongoing management actions on the atoll; he also shared his excellent photographs. We thank those who provided species identifications: Neil Evenhuis (flies), Karl Magnacca (wasps), and Jai-Wei Tay (UH-M), who kindly identified the Asian subterranean termite. We thank Mashuri Waite, Forest and Kim Starr, Kenneth Puliafico, Leyla Kaufman, Alex Wegmann, Danko Taboroši, Levi Gray, Diane Elam, and especially, Joel Helm and James Stanford (PRSC) for their thoughtful reviews and helpful comments on this report. Finally, this project would not have been possible without the close and enjoyable collaboration of the survey team, which included authors Adam Backlin and Stacie Hathaway (USGS-WERC), Jim Jacobi (U.S. Geological Survey Pacific Island Ecosystems Research Center), and Bob Peck (Hawai‘i Cooperative Studies Unit at the University of Hawai‘i at Hilo).
A. Introduction
Wake Atoll (generally referred to hereafter as “Wake”) is part of the Gilbert-Marshall Island chain in the Pacific Ocean, about 3,500 kilometers (km) west of the Hawaiian Islands, 2,600 km east of Guam, 3,200 km southeast of Japan, and 570 km north of Bokak Atoll in the Republic of the Marshall Islands (Bryan, 1959; U.S. Air Force, unpub. data, 2017). Wake is one of the most isolated terrestrial islands in the Pacific (fig. A1; U.S. Air Force, unpub. data, 2017). Wake consists of three islets: (1) Peale, (2) Wake, and (3) Wilkes, arranged in a V-shaped pattern around a central lagoon (fig. A2; Bryan, 1959). Wake is a low atoll with an average elevation of about 4 meters (m), maximum elevation of about 6.4 m above sea level, and a total land area of about 7 square kilometers (km2; U.S. Air Force, unpub. data, 2008). The climate is tropical maritime with little annual temperature variation (U.S. Air Force, unpub. data, 2008). Mean annual temperatures range from 24.4 to 28.3 degrees Celsius (°C), with an annual maximum of 35 °C and a minimum of 20 °C. Rainfall averages about 890 millimeters (mm) per year (Weatherbase, 2020). Together, high temperatures and low rainfall generally keep Wake in a state of drought (U.S. Air Force, unpub. data, 2008). Frequent tropical storms and typhoons generating high winds and waves can cause considerable damage to vegetation and infrastructure (U.S. Air Force, unpub. data, 2008). Wake consists of porous coral rubble and limestone with organic matter in vegetated areas (U.S. Air Force, unpub. data, 2008). Despite low endemism and biodiversity in general, Wake and other atolls protect various natural resources, including several terrestrial and marine natural resources, such as plants, seabirds, shorebirds, lizards, and sea turtles (Engilis and Naughton, 2004; U.S. Fish and Wildlife Service, 2005; Thaman, 2016).
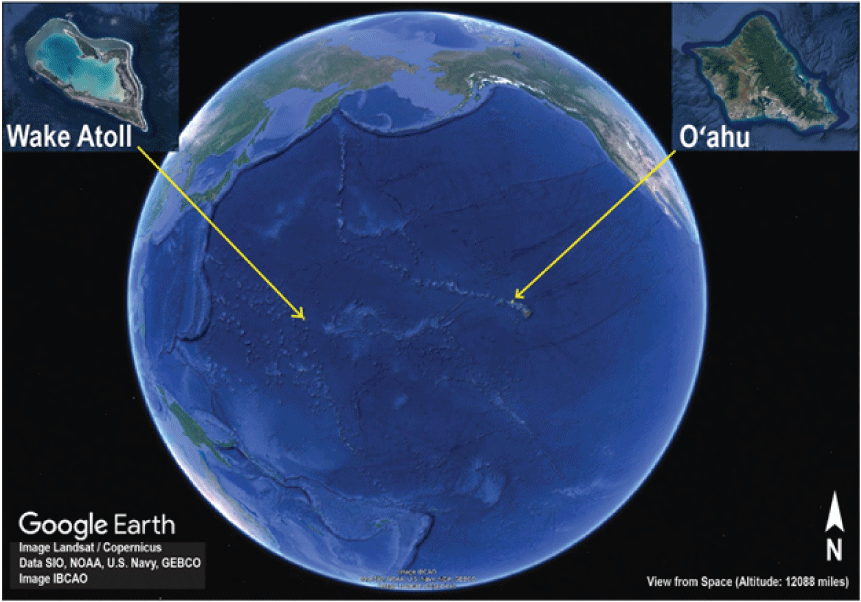
Location of Wake Atoll (Google Earth image, 2016).

Wake Atoll with habitat mapping units and selected sites referred to in the report (U.S. Air Force, WorldView 3 image, taken October 2015).
There is no prehistoric evidence that Wake was populated by pre-European Pacific peoples. Heinl (1947) provides an account of the pre-war history of Wake from 1568 to 1941, and additional historical context is contained in the Wake Integrated Cultural Resources Management Plan (Verhaaren and Kullen, unpub. data, 2014) and the Wake Integrated Natural Resources Management Plan (INRMP; U.S. Air Force, unpub. data, 2017). The brief history that follows is summarized from these documents. Wake was discovered in 1568 by Spanish explorers, although credit is given to British Captain William Wake, who rediscovered the island more than 200 years later in 1796. Wake was explored by U.S. Navy Commander Charles Wilkes and naturalist Titian Peale in 1841. The United States formally took possession in 1899. There are reports of several shipwrecks and otherwise limited visitations until the Japanese began landing to harvest bird feathers and fish for shark fins. A group of Japanese castaways were marooned on the atoll at one point. Remaining Japanese camps were abandoned by 1922. Most early zoological and botanical observations are from the Smithsonian’s Tanager Expedition, which carried out a biological reconnaissance at Wake in 1923. The U.S. Navy was given jurisdiction over Wake in 1934 and gave permission for Pan American Airlines (Pan Am) to begin constructing facilities to support weekly trans-Pacific flights. In 1938, the Navy began plans for an outlying military base; however, construction did not begin until January 1941. Construction was not yet completed when the Japanese invaded and overran the island in December 1941 and occupied Wake for the rest of World War II. During the war, the Japanese continued to build many structures underground and behind embankments for protection against repeated bombing. After Japanese surrender in 1945, the atoll returned to U.S. possession and was placed under the jurisdiction of the U.S. Navy. Later, the civil administration was given to what is now known as the Federal Aviation Administration (FAA). Military Air Transport Services and, later, Military Airlift Command provided service to transient U.S. Air Force (USAF) aircraft while at Wake, and Pan Am and other airlines reestablished commercial airline services. During that period, population rose to roughly 2,000 people at the atoll, and an elementary school was constructed. Additional botanical and bird surveys were carried out during this period. In 1972, when long-range jet aircraft reduced the need for Wake as a refueling stop, the FAA transferred jurisdiction to the USAF until 1994. After this time, Wake was administered by the U.S. Army for missile defense and then transferred back to the USAF in 2002. On January 6, 2009, by Presidential Proclamation 8336, Wake Atoll was included in the establishment of the Pacific Remote Islands Marine National Monument. The Secretary of the Interior, in consultation with the Secretary of Commerce, is responsible for the management of the monument. On January 16, 2009, through Secretary Order 3284, the Secretary of the Interior delegated management of the monument to the U.S. Fish and Wildlife Service (USFWS). In accordance with Proclamation 8336, this order (3284) states that Wake is under management by the USAF under the 1972 agreement with the Secretary of the Interior (Code of Federal Regulations 32 Part 935) until the agreement is terminated. The USFWS manages the areas surrounding Wake Atoll from the mean low water line out to 50 nautical miles as part of the National Wildlife Refuge System. Emergent lands are managed by the USAF and used for contingency deployments, an emergency landing facility, and fuel storage. With these activities, construction and maintenance at Wake have continued. In addition, there are currently (2019) regular flights to and from the atoll that carry passengers and supplies. Oceangoing barges bring the bulk of materials and supplies to the atoll and transport used equipment and materials off island.
This history is important for understanding how Wake and its natural resources have been affected over time and illustrates an array of past and current (2019) pathways for invasive species. Non-native species have the potential to be invasive, defined by Executive Order numbers 13112 and 13751, as “species whose presence has caused harm or may cause harm to environmental or human, animal, or plant health” (National Invasive Species Council, 2008). Invasive species are well known to be important factors in the decline of unique natural communities, species, and ecological processes (Vitousek, 1990; numerous papers in Veitch and Clout, 2002; Engilis and Naughton, 2004). Per the Sikes Act, the USAF currently (2019) uses INRMPs to manage and protect natural resources on installations. The INRMP that addresses Wake includes components that cover biosecurity and pest management. These are long-term planning documents to guide Department of Defense (DOD) natural resource managers in the management of natural resources to support installation missions while protecting and enhancing resources for multiple uses and biological integrity (U.S. Department of Defense, 2018; U.S. Air Force, 2020). The initial plan introduced the goal to “bring together and integrate all management activities in a way that sustains, promotes, and restores the health and integrity of ecosystems and that enhances the human environment on Wake Atoll” (Foothill Engineering Consultants, Inc., written commun., 2000). The 2008 INRMP identified the need for an invasive species risk assessment (U.S. Air Force, written commun., 2008).
Invasive/pest species are recognized as one of the greatest threats to ecosystems and economies (Vitousek and others, 1997; Warziniack and others, 2021). Biosecurity is thus a concern at several scales from global to local, and to address this issue, prevention and control policies have been and continue to be improved at several levels of government (Ricciardi and others, 2020; Rawluk and others, 2021). A biosecurity plan is an effective tool for identifying and addressing non-native, potentially invasive species problems and concerns (Matos and others, 2018). In 2012, the USAF, with support from private consultants, authored the “Wake Island Biosecurity Management Plan” (U.S. Air Force, unpub. data, 2012). The plan re-defined the container requirements and other elements of USAF shipping to the atoll. The biosecurity plan was updated in 2015 (U.S. Air Force, unpub. data, 2015) and incorporated into the 2017 INRMP as a component plan. The current (2015) biosecurity plan retains a rodent focus. However, some components of the intervention measures within it have potential for inhibiting or intercepting invasive species other than rodents. The INRMP calls for this plan to be updated periodically. In 2017, the U.S. Air Force issued funds to the U.S. Geological Survey (USGS) to update the biosecurity plan, create a current (2019) flora and fauna species identification index, and do container evaluations for the presence of potential invasives. The current (2019) biosecurity protocols used for prevention were evaluated (S.A. Hathaway, C.S. Brehme, and R.N. Fisher, U.S. Geological Survey; J.C. Molden, U.S. Fish and Wildlife Service; R. Peck, Hawai`i Cooperative Studies Unit, University of Hawai`i at Hilo; and K.R. Rex, National Oceanic and Atmospheric Administration, unpub. data, 2022), and new biodiversity surveys were completed for terrestrial vegetation and arthropods and included the first formal reptile surveys. Results from field efforts add to existing knowledge and may identify new species arrivals to Wake.
One goal of this project was to update and compile established species information for the atoll and create species identification guides for the three taxonomic groups surveyed. We made these flora and fauna species identification guides by compiling results of the recent (2019) and historical surveys. The guides can be used as resident desktop references, as a baseline for assessing future natural resource surveys, and to assist with guiding management actions. We refer herein to biosecurity and integrated pest management plan materials, which we created simultaneously to inform current (2019) biosecurity and to identify some of the top invasive species at Wake (Hathaway and others, 2022). This study was done in cooperation with the USAF, and surveys were performed for the 611th Civil Engineer Squadron Natural Resources Program, ACES PROJECT #YGFZ170002 under agreement number F2MUAA7116GW02 between the USAF and the USGS-Western Ecological Research Center.
A. Methods
Species Identification Guides
We compiled data specific to Wake from museums, herbariums, and published and unpublished literature; we also interviewed people with local knowledge to understand the status and distribution of flora and fauna known historically to exist on the atoll.
Wake, Wilkes, and Peale Islets have been separated into habitat management units (HMUs), which were delineated to assist in the creation of natural resources management actions and approaches (U.S. Air Force, unpub. data, 2017. We performed flora and fauna surveys broadly across HMUs on all three islets as well as in focused areas most likely to be vulnerable to invasive species incursions (for example, the marina and associated area within HMU-11; areas with concentrated populated buildings, such as in HMU-58; and cargo container unloading and storage areas within HMU-65; fig. A2). Field surveys were carried out at Wake between May 24 and June 7, 2019. For the specific methods employed and details about plants, arthropods, and reptiles, see chapters B, C, and D, respectively.
A. Results
We identified more than 450 species of terrestrial plants, arthropods, and reptiles that have been recorded at Wake (table A1, see chapters B, C, and D for plants, arthropods, and reptiles, respectively). There have been 229 vascular plant species documented during past and current (2019) surveys; 20 are considered native, and 1 is endemic to Wake, whereas 209 are nonnative (table A1). In 2019, a total of 153 plant species were recorded: 19 native and 134 nonnative; 15 were newly reported nonnative species. One native and 75 nonnative plant species previously reported at Wake were not observed in 2019.
Between 221 and 237 total arthropod species have been recorded at Wake. Varying levels of identification impeded comparisons across surveys; therefore, an exact assessment of how many species in total have actually been recorded could not be made. Also, given that reasonable assessment of species provenance has not been possible (see chapter C, “Wake Atoll 2019 Arthropod Species Survey Report and Field Guide”), we could not clearly distinguish which species should be considered native or nonnative to Wake. Between 140 and 156 species had not previously been reported for Wake, and between 48 and 64 species previously recorded were not observed in 2019.
Nine reptile species have been reported at Wake over time; four of these we consider to be native. Of these reptile species, we observed three native and one nonnative lizard species in 2019. We also obtained photographic evidence of one nonnative snake species in 2017. We did not directly detect this species ourselves in the field in 2019.
A. Discussion
As summarized herein, there are 20 terrestrial plant and 4 terrestrial reptile species considered native to Wake. Although there are undoubtedly terrestrial arthropods that are native to Wake, determining with confidence which species are native is beyond the scope of this study. We deduce there have been up to 451 nonnative terrestrial plant (209), arthropod (up to 237), and reptile (5) species recorded on Wake (table A1). About 7 percent (15) of all nonnative plants and up to about 70 percent (156) of arthropod species detected in 2019 had not been previously recorded. This finding may indicate that introductions of new species, particularly arthropods, are ongoing. However, given that many of these species are likely uncommon and patchily distributed, and given the brevity of these surveys, some or even many of these species may have been present previously but not observed. Conversely, it also is possible that some species detected during our surveys were newly introduced and had not yet established or been able to establish. Likewise, approximately 39 percent of nonnative plant species and up to 29 percent of arthropod species were previously recorded but not observed in 2019. Previously unrecorded species of reptiles were not detected in 2019. However, the previously recorded common house gecko (Hemidactylus frenatus), along with various plant seeds and arthropods detected in shipping containers sent to Wake in 2018 (S.A. Hathaway, U.S. Geological Survey; J.C. Molden, U.S. Fish and Wildlife Service; and K.R. Rex, National Oceanic and Atmospheric Administration, unpub. data, 2022), indicates that continued introductions of some established species does happen.
Because of time constraints, some HMUs were less thoroughly assessed than others, and not all HMUs were examined. However, surveys were as representative as time and access permitted; surveying broadly across the atoll, including multiple HMUs on each of the three islets, and focusing on areas we considered most likely to harbor new arrivals. The compilations of historical and 2019 survey data presented here provide a current (2019) baseline for making decisions regarding research, monitoring, planning, and management. These results include examples of recent (since last documented according to sources we report in the chapters herein) changes in biodiversity that point to the importance of robust biosecurity practices.
Table A1.
Summary of species recorded during surveys by U.S. Geological Survey on Wake Atoll in 2019 (from chapters B, C, and D for plants, arthropods, and reptiles, respectively).[No., number; sp., species; unk, unknown; —, not determined]
A. References Cited
-
Bryan, E.H., Jr., 1959, Notes on the geography and natural history of Wake Island: Atoll Research Bulletin, v. 66, p. 1–22. [Available at https://doi.org/10.5479/si.00775630.66.1.]
-
Engilis, A., Jr., and Naughton, M., 2004, U.S. Pacific Islands regional shorebird conservation plan—U.S. shorebird conservation plan: Portland, Oreg., U.S. Fish and Wildlife Service, 17 p.
-
Hathaway, S.A., Jacobi, J.D., Peck, R., and Fisher, R.N., 2022, Updates for Wake Atoll biosecurity management, biological control, survey, and management, and integrated pest management plans: U.S. Geological Survey Open-File Report 2022–1067, 56 p., accessed August 26, 2022, at https://doi.org/10.3133/ofr20221067.
-
Heinl, R.D., Jr., 1947, The defense of Wake: Washington, D.C., Historical Section, Division of Public Information, Headquarters, U.S. Marine Corps, 75 p.
-
Matos, J., Little, A., Broome, K., Kennedy, E., Méndez Sánchez, F.A., Latofski-Robles, M., Irvine, R., Gill, C., Espinoza, A., Howald, G., Olthof, K., Ball, M., and Boser, C.L., 2018, Connecting island communities on a global scale—Case studies in island biosecurity: Western North American Naturalist, v. 78, no. 4, p. 959–972. [Available at https://doi.org/10.3398/064.078.0432.]
-
National Invasive Species Council, 2008, 2008–2012 National invasive species management plan: Washington, D.C., National Invasive Species Council, 35 p.
-
Rawluk, A., Beilin, R., and Lavau, S., 2021, Enacting shared responsibility in biosecurity governance—Insights from adaptive governance: Ecology & Society, v. 26, no. 2, art. 18, accessed July 25, 2021, at https://doi.org/10.5751/ES-12368-260218.
-
Ricciardi, A., Iacarella, J.C., Aldridge, D.C., Blackburn, T.M., Carlton, J.T., Catford, J.A., Dick, J.T.A., Hulme, P.E., Jeschke, J.M., Liebhold, A.M., Lockwood, J.L., MacIsaac, H.J., Meyerson, L.A., Pyšek, P., Richardson, D.M., Ruiz, G.M., Simberloff, D., Vilà, M., and Wardle, D.A., 2020, Four priority areas to advance invasion science in the face of rapid environmental change: Environmental Reviews, v. 29, no. 2, p. 119–141, accessed December 1, 2021, at https://doi.org/10.1139/er-2020-0088.
-
Thaman, R.R., 2016, Atolls of the tropical Pacific Ocean—Wetlands under threat: Netherlands, Springer Science+Business Media, Dordrecht, p. 1–25.
-
U.S. Air Force, 2020, Air Force Manual 32-7003, Environmental conservation: U.S. Air Force, 127 p. [Available at https://static.e-publishing.af.mil/production/1/af_a4/publication/dafman32-7003/dafman32-7003.pdf.]
-
U.S. Department of Defense, 2018, Department of Defense 4715.03—Integrated natural resources management plan (INRMP) implementation manual: U.S. Department of Defense, 31 p. [Available at https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodm/471503m.pdf?ver=2018-11-13-125658-050.]
-
U.S. Fish and Wildlife Service, 2005, Regional seabird conservation plan, Pacific region: Portland, Oreg., U.S. Fish and Wildlife Service, Migratory Birds and Habitat Programs, Pacific Region, 264 p.
-
Veitch, C.R., and Clout, M.N., eds., 2002, Turning the tide—The eradication of invasive species, in International Conference on Eradication of Island Invasives, Auckland, New Zealand, 2001, Proceedings: Auckland, New Zealand, International Union of Conservation of Nature, 414 p.
-
Vitousek, P.M., 1990, Biological invasions and ecosystem processes—Towards an integration of population biology and ecosystem studies: Oikos, v. 57, no. 1, p. 7–13. [Available at https://doi.org/10.2307/3565731.]
-
Vitousek, P.M., D’Antonio, C.M., Loope, L.L., Rejmanek, M., and Westbrooks, R., 1997, Introduced species—A significant component of human-caused global change: New Zealand Journal of Ecology, v. 21, p. 1–16.
-
Warziniack, T., Haight, R.G., Yemshanov, D., Apriesnig, J.L., Holmes, T.P., Countryman, A.M., Rothlisberger, J.D., and Haberland, C., 2021, Economics of invasive species, in Poland, T.M., Patel-Weynand, T., Finch, D.M., Miniat, C.F., Hayes, D.C., and Lopez, V.M., eds., Invasive species in forests and rangelands of the United States—A comprehensive synthesis for the United States Forest Sector: Cham, Switzerland, Springer, p. 305–320. [Available at https://doi.org/10.1007/978-3-030-45367-1_14.]
-
Weatherbase, 2020, Wake Island, Oceania: Weatherbase online database, accessed December 23, 2020, at http://www.weatherbase.com/weather/weather.php3?s=54219&cityname=Wake-Island-Wake-Island-Oceania.
B. Introduction
The native flora and plant communities reported on Wake Atoll are relatively simple (Fosberg, 1959; Mueller-Dombois and Fosberg, 1998). The extreme isolation of this location has limited the number of plant species able to colonize the very small land surface naturally. The 21 species of vascular plants considered native to Wake are just a small subset of the flora detected across the pan-tropical Pacific islands (Mueller-Dombois and Fosberg, 1998), and one of these, Gossypium stephensii, is endemic to this atoll (Gallagher and others, 2017). Plant diversity on Wake is limited by the relatively uniform dry-arid habitat due to a combination of the porosity of the coral-based substrate (Bryan, 1959) and low annual precipitation, which is approximately 1,015 millimeters (mm) per year (Fosberg, 1959).
In addition to the native flora, many plants have been either purposely or accidentally introduced to the atoll by humans. Although most of these nonnative plants are detected only in cultivation as household or yard ornamentals or in gardens, many introduced species have become naturalized, and a few are considered to be invasive, posing actual or potential threats to maintaining the natural flora and vegetation (D.R. Herbst, B.P. Bishop Museum, unpub. data, 1998; Mueller-Dombois and Fosberg, 1998; U.S. Air Force, unpub. data, 2017).
During summer 2019, a 2-week field survey was done on Wake to assess the status of the plant species and plant communities reported there. This survey was part of a larger project to document the current (2019) status of plant communities, terrestrial reptiles, and arthropods on the atoll and to provide input into the creation of an expanded integrated pest management and biosecurity plan for the Department of Defense.
Specific objectives of the vegetation survey included the following:
-
1. compile lists of plant species that have been collected or recorded on Wake during previous surveys;
-
2. complete a new field survey to determine the current (2019) status of plant species detected on the atoll; and
-
3. produce a list and photograph key describing the plant species previously and currently (2019) recorded from Wake, including information on taxonomy, status, distribution, and potential threats from nonnative species.
B. Methods
Before initiating field work, lists of vascular plant species that had been recorded during previous surveys on Wake were compiled into a relational database. Information in the database includes data source reference and survey dates, current (2019) taxonomic nomenclature, status in the flora (native or introduced), status of the nonnative plants (cultivated or naturalized), and location information on each species on the three islets. Where needed, taxon names were updated to their currently (2019) accepted nomenclature following Roskov and others (2019).
The field survey was done on Wake from May 24 to June 7, 2019. During this time, all areas and habitat types on the three islets were selectivity visited, and plant species were recorded by site and habitat type (fig. B1). Generally, this process involved walking to an arbitrarily chosen representative point within a plant community and documenting all plant species detected in that local vicinity. The Global Positioning System (GPS) coordinates for the site were recorded, as well as information on habitat conditions and other plant communities in that location. Photographs were taken of representative individuals for the recorded species. Each photograph included a date and time stamp, as well as GPS coordinates recorded in decimal degrees so species locations could be accurately mapped.
Information on plants previously recorded on Wake, as well as during the 2019 survey, is presented in tables B1–B3 and appendix B1. The status of species in these tables is identified as follows: “cultivated”—species that were only detected in cultivation and were not reproducing in the wild; “alien invasive”—introduced species that were reported to be established in the wild and considered to be invasive elsewhere (U.S. Forest Service, 2018; Florida Invasive Species Council, 2019; Hawaiʻi-Pacific Weed Risk Assessment, 2019; International Union for Conservation of Nature, 2019; U.S. Department of Agriculture, 2019); “alien naturalized”—species that were reported to be established in the wild but not considered to be invasive; “native”—species that were identified as naturally detected on Wake during previous surveys (Christophersen, 1931; Bryan, 1959; Fosberg, 1959; Fosberg and Sachet, 1969; D.R. Herbst, Bernice Pauahi Bishop Museum, unpub. data, 1998); and “endemic”—species unique only to Wake. In table B2, an introduced species is identified as a threat to biodiversity on Wake if it was reported to be established in the wild and shown to be invasive elsewhere. Also, in table B2, the risk an introduced species poses to biodiversity on Wake ranges from high to medium to low based on the previously mentioned published risk assessments. However, in several cases, the risk category was modified for Wake based on the fact that (1) a species was only detected in cultivation and unlikely to survive outside that level of care; (2) a species with a high risk factor elsewhere currently (2019) has less potential for spread and effects on Wake due to a lack of dispersal agents (for example, seed- or fruit-eating birds); or (3) a species is only in cultivation, and the conditions for its establishment on Wake are minimal due to lack of habitat (for example, waterlily or water hyacinth). Management feasibility in table B2 is identified as “high” for species that are considered to be invasive but not yet widespread and “medium” for species that are invasive but more widespread and require greater effort for control or eradication. The actual cost and capacity needed to control a species will vary depending on the distribution of the species, availability of effective control tools or protocols, and regenerative capability of the species, including persistence of the seedbank. In table B3, species distribution is coded based on the extent of the species in the field in the 2019 survey (widespread or local), and on an estimate of relative abundance (high, medium, low) for each species at that time.
The initial plant species list photo-key that was produced prior to the field survey was updated to include additional species that were identified during the 2019 survey. This photograph key (see the last section of this chapter) includes up to four images of each species and enough detail to generally allow an observer to identify the species in the field. Additionally, each taxon page includes the scientific and common names for the species, its plant family, life form, and status on Wake, as well as information on its past and current (2019) distribution. Species recorded in tables B1–B3, appendix B1, and the “Plant Field Guide to Wake Atoll” section represent only taxa that had clear documentation of their presence on Wake, primarily based on records provided by Christophersen (1931), Bryan (1959), Fosberg and Sachet (Fosberg, 1959; Fosberg and Sachet, 1969), D.R. Herbst Bernice Pauahi Bishop Museum (unpub. data, 1994, 1998), and the 2019 survey. However, to reduce the Field Guide’s size, only species recorded by Herbst in his two surveys and species recorded during the 2019 survey are documented in the photo-key. Common vegetable garden plants and many household ornamentals were also omitted from the Field Guide unless they had high potential to be invasive outside of cultivation.
The results of the current (2019) survey are used to identify several invasive species that resource managers may use on Wake as priorities for integrated pest management. These identifications include some species that already exist on Wake Atoll, as well as others that are not yet reported there but pose a risk to the native ecosystems if they become established (table B4). Descriptions of these species are included in the “Discussion” section, and potential management options for these species are summarized in the biosecurity and integrated pest management plan materials document (Hathaway and others, 2022).
B. Results
A total of 229 vascular plant species have been documented on Wake during previous reporting and 2019 surveys (tables B1–B3; appendix B2; “Plant Field Guide to Wake Atoll” section). Although several naturalists have visited Wake since its discovery and described some of the plants and plant communities detected there (Christophersen, 1931; Bryan, 1959), the first systematic surveys of the atoll were done by Fosberg and Sachet (Fosberg, 1959; Fosberg and Sachet, 1969). Fosberg and Sachet (Fosberg, 1959; Fosberg and Sachet, 1969) recorded 112 species, 18 of which were native and 94 introduced (table B1). Herbst also surveyed this area in 1994 and 1998 and reported 169 species, 18 native and 151 introduced. In both surveys, most of the introduced species were detected only in cultivation.
During the 2019 survey, 153 plant species were recorded. Of this species total, 19 were native and 134 introduced (tables B1–B3). These plant species numbers are similar to those detected by Herbst in 1994 and 1998, although he recorded many more cultivated species than were detected in the 2019 survey (table B1).
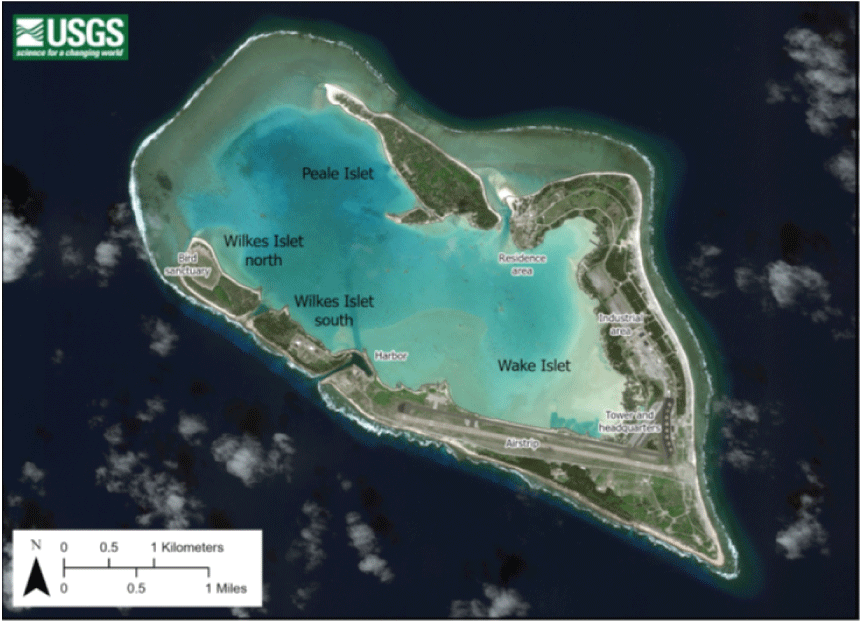
Wake Atoll, including the three islets around the lagoon and selected sites referred to in the report. (WorldView 3 image, taken October 2015).
Most of the plants recorded during the 2019 survey were detected on Wake Islet (151 species), with lesser amounts detected on Peale (56) and Wilkes (34) Islets (table B3; fig. B2). One of the native species, Heliotropium anomalum (fig. B3), is quite rare and detected in only two locations on Wake Islet. The only endemic species to this atoll, Gossypium stephensii (fig. B4), was detected abundantly on Wake and Peale Islets and on the south part of Wilkes Islet, northwest of the harbor. This species seems to be a successful colonizer of open, previously disturbed habitats (Gallagher and others, 2017). There were 15 species detected during the 2019 survey that had not been previously documented by Fosberg and Sachet (Fosberg, 1959; Fosberg and Sachet, 1969) or D.R. Herbst (Bernice Pauahi Bishop Museum, unpub. data, 1994, 1998); however, many of these species were included in an unpublished species list compiled by M. Waite while he was working on Wake (J. Gilardi, Island Conservation, written commun. May 29, 2019).

Location of sites on Wake Atoll where plant species were recorded during the 2019 field survey. (WorldView 3 image, taken October 2015).

Heliotropium anomalum growing in open habitat on the northeast side of Wake Islet. (Photograph by J. Jacobi, U.S. Geological Survey, 2019).
Areas sampled in 2019 included most of the Habitat Management Units described in the 2017 Wake Integrated Natural Resources Management Plan (U.S. Air Force, unpub. data, 2017). Voucher specimens were collected for 44 of the species detected during the survey in 2019 to aid with their identification or to document new records (appendix B3). These specimens are deposited in the herbarium at the B.P. Bishop Museum in Honolulu, Hawai‘i.

Native species of cotton (Gossypium stephensii) that is endemic to Wake Atoll. (Photograph by J. Jacobi, U.S. Geological Survey, 2019).
B. Discussion
Current (2019) Status of Plants and Plant Communities
The low topographic range, meager precipitation, and biogeographic isolation of Wake have all contributed to a flora that is relatively simple, capable of natural recovery from disturbance but also susceptible to change. The dynamic nature of the flora on Wake is a result of both natural perturbations, such as hurricanes or tsunamis, and other factors that include landscape alteration, human disturbance, and effects from introduced species. Primary documented invasive species effects are from introduced rats (Rattus exulans and R. tanezumi; Griffiths and others, 2014; U.S. Air Force, unpub. data, 2017) and from a select number of ecosystem-altering plant species (U.S. Air Force, unpub. data, 2017). It is currently (2019) unclear how introduced invertebrates have affected the native flora and plant communities.
The 2019 survey reported that the dominant native plant communities generally appeared to be maintaining their structure and composition in the lesser disturbed areas throughout the atoll. These communities are relatively simple and include (1) a widespread Heliotropium foertherianum woodland with a native grass and shrub understory (fig. B5); (2) a Pemphis acidula (fig. B6) shrubland that primarily grows along the shoreline, particularly on the lagoon sides of the islets; (3) a mixed Heliotropium woodland with Cordia subcordata (fig. B7) and Pisonia grandis trees (fig. B8); (4) a shrubland dominated by Scaevola sericea (fig. B9), currently (2019) limited in distribution primarily on the southeast tip of Wake Islet; (5) a small community dominated by Sesuvium portulacastrum (fig. B10) that grows around the edges of the few small wetland areas on Wake and Peale Islets; and (6) a very narrow fringe of native grass, primarily Lepturus repens (fig. B11), and scattered native shrubs that form a strand community just above the water’s edge around all the islets. Additionally, there is a unique low-stature native shrubland in the area referred to as the “Bird Sanctuary” on the northwestern part of Wilkes Islet. The area is dominated by the native shrubs Tribulus cistoides and Lepidium bidentatum, but also includes Sida fallax, two Boerhavia species, and the herb Portulaca lutea (fig. B12). This area has been mechanically mowed repeatedly to provide habitat for several species of ground-nesting seabirds (U.S. Air Force, unpub. data, 2017), and native plant species there appear to be able to tolerate this level of disturbance and continue to dominate the vegetation.

Open Heliotropium foertherianum woodland on Peale Islet. (Photograph by J. Jacobi, U.S. Geological Survey, 2019).

Flowers and immature fruit of Pemphis acidula, which forms a dense shrub community, particularly around the lagoon sides of Wake, Wilkes, and Peale Islets. (Photograph by J. Jacobi, U.S. Geological Survey, 2019).
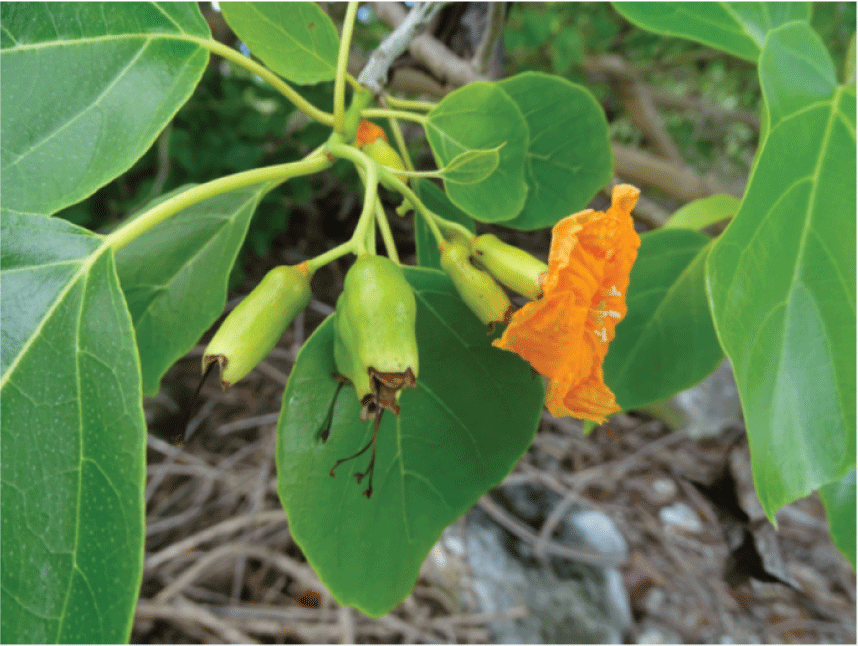
Flower of the tree Cordia subcordata, which grows in a mixed woodland-shrub community with Heliotropium foertherianum and Pisonia grandis on Wake Atoll. (Photograph by J. Jacobi, U.S. Geological Survey, 2019).
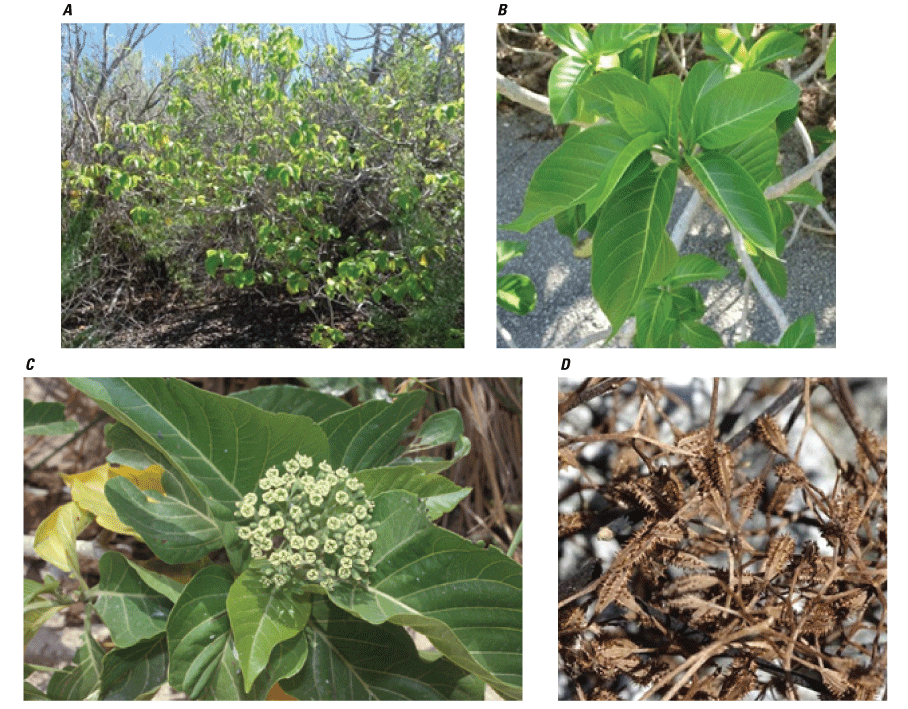
Pisonia grandis and its A, habitat; B, leaves; C, flowers; and D, sticky seed pods. (Photographs A, B, and D by J. Jacobi, U.S. Geological Survey, 2019, and photograph C by Forest and Kim Starr, Starr Environmental, 2018).

A native shrubland dominated by Scaevola sericea was probably more widely distributed prior to human disturbance; it is now detected in a few locations, primarily on the southeastern tip of Wake Islet. (Photograph by J. Jacobi, U.S. Geological Survey, 2019).

Sesuvium portulacastrum, a succulent herb that forms dense mats around the edges of brackish wetlands on Wake and Peale Islets. (Photograph by J. Jacobi, U.S. Geological Survey, 2019).

The native grass, Lepturus repens, is detected in the strand vegetation on Wake Atoll and in more inland sites, particularly on Peale Islet, where it forms a dense grass cover under an open Heliotropium foertherianum woodland. (Photograph by J. Jacobi, U.S. Geological Survey, 2019).

View of the mowed Bird Sanctuary area on the north part of Wilkes Islet; this community is dominated by several native plants, including Tribulus cistoides and Lepidium bidentatum. (Photograph by J. Jacobi, U.S. Geological Survey, 2019).
The rest of the landscape on the three islets is comprised of a variety of communities that are in various stages of disturbance or recovery from disturbance but primarily dominated by introduced plant species. These communities include grass-dominated areas that are frequently mowed or cleared, such as along roadsides and the sides of the airstrip or in the industrial area on Wake Islet and the harbor area at the southwest tip of Wake. Also included in these communities, are large areas on all the islets that are dominated primarily by ironwood (Casuarina equisetifolia; fig. B13), with little growing under its dense canopy and thick layer of litter on the ground. Additionally, many of the roadsides beyond the regular mowing zone have been invaded by numerous introduced shrubs, including Pluchea carolinensis, Stachytarpheta jamaicensis, Bidens alba, and several introduced grasses.

Large Casuarina equisetifolia (ironwood) tree growing in the open near the residential area on Wake Islet. (Photograph by J. Jacobi, U.S. Geological Survey, 2019).
Most of the introduced plant species detected during the previous and current (2019) surveys on Wake were cultivated plants that were grown either as ornamentals around the residence areas or in vegetable gardens (table B2; figs. B14–B23). The variation in numbers of cultivated species recorded during the three surveys reflects what the residents grew at that time. From 1969 to 2019, there was a slow increase in the number of naturalized and invasive species recorded on Wake (table B1). When all the survey results are combined, 67 introduced species were reported to be naturalized, and 33 of these species are considered invasive in other areas of the Pacific (U.S. Forest Service, 2018; Florida Invasive Species Council, 2019; Hawaiʻi-Pacific Weed Risk Assessment, 2019; U.S. Department of Agriculture, 2019).

Coccoloba uvifera (sea grape) trees are becoming established in several locations on Wake and Peale Islets and have the potential to rapidly spread and dominate the native vegetation if not controlled. (Photograph by J. Jacobi, U.S. Geological Survey, 2019).

Cenchrus setaceus has aggressively invaded dry lowland habitats in Hawai‘i, dominating the vegetation and posing a high risk for wildfire. (Photograph by J. Jacobi, U.S. Geological Survey, 2019).

Clusia rosea is a small tree that grows along dry shorelines and lowland habitats in many areas across the Pacific. (Photograph by Forest and Kim Starr, Starr Environmental, 2019).

Lantana camara is a small woody shrub that has been able to dominate lowland dry and mesic habitats in Pacific Island ecosystems, such as those in Hawai‘i. (Photograph by J. Jacobi, U.S. Geological Survey, 2019).

The aggressive, invasive vine, Neonotonia wightii, has the ability to smother the vegetation in lowland habitats, as seen in many areas in Hawai‘i. (Photograph by Forest and Kim Starr, Starr Environmental).

Black noddy tern (Anous minutus) perching on a branch of a Casuarina equisetifolia (ironwood) tree. (Photograph by J. Jacobi, U.S. Geological Survey, 2019).

Closeup of foliage and cones of Casuarina equisetifolia. (Photograph by J. Jacobi, U.S. Geological Survey, 2019).

The introduced sandbur grass (Cenchrus echinatus) is becoming established in many sites on Wake and Peale Islets. (Photograph by J. Jacobi, U.S. Geological Survey, 2019).

Flowers, immature seed pods, and foliage of the introduced invasive tree Leucaena leucocephala, which is becoming established in several locations on Wake and Peale Islets. (Photograph by J. Jacobi, U.S. Geological Survey, 2019).

The introduced vine Passiflora foetida var. hispida is currently (2019) detected on Wake Islet and the southeastern part of Wilkes Islet just north of the harbor inlet and storage tanks. (Photograph by John Gilardi, Island Conservation, 2018).
Table B1.
Summary of plant species recorded on Wake Atoll in the 2019 survey or during previous surveys by Fosberg and Sachet (1969) and D.R. Herbst (B.P. Bishop Museum, unpub. data, 1998).[—, not applicable; I+E, indigenous plus endemic]
Table B2.
List and status of plant species recorded in 2019 or during previous botanical surveys on Wake Atoll.[Taxonomy follows Roskov and others (2019). Status: Cul, cultivated; A-N, alien naturalized; A-I, alien invasive; E, endemic; Nat, native; A, alien. Impact Description: B, biodiversity; n/a, native species so no impact. Wake risk assessment: H, high; M, medium; L, low; n/a, native species. Management feasibility: H, high, M, medium. Published risk-assessment status: High risk, High risk invasive species; No Assessment, No risk assessment conducted. Published risk-assessment reference: HPWRA, Hawaiʻi-Pacific Weed Risk Assessment, 2019 (www.hpwra.org); FISC database, Florida Invasive Species Council, 2019 (https://www.fleppc.org/); PIER, Pacific Island Ecosystems at Risk, U.S. Forest Service, 2018 (http://www.hear.org/pier/); USDA-APHIS, U.S. Department of Agriculture Animal and Plant Health Inspection Service; Wake 2019, Wake 2019 Survey. Published risk: N, no assessment; L, low risk; H, high risk; E, evaluate; n/a, not applicable. Abbreviation: —, not determined]
Table B3.
Distribution of plant species recorded in 2019 or during previous botanical surveys on Wake Atoll.[Taxonomy follows Roskov and others (2019). Status: Cul, cultivated; A-N, alien naturalized; A-I, alien invasive; A, alien; E, endemic; Nat, native. Distribution level: W: high, widespread distribution with high abundance; W: mod, widespread distribution with moderate abundance; W: low, widespread distribution with low abundance; L: mod to high, local distribution with moderate to high abundance; L: mod, local distribution with moderate abundance; L: low, local distribution with low abundance; Unknown: not recorded in 2019 survey. Historical distribution references: Fosberg and Sachet, 1969. Wake Island vegetation and flora, 1961–63: Atoll Research Bulletin 1231–15; D.R. Herbst, Bernice Pauahi Bishop Museum, unpub. data, 1998. Abbreviations: X, species was detected; —, not applicable; Y, yes; C, collected]
Table B4.
List of invasive plant species that could become established on Wake Atoll if introduced.[—, not applicable]
B. References Cited
-
Bryan, E.H., Jr., 1959, Notes on the geography and natural history of Wake Island: Atoll Research Bulletin, v. 66, p. 1–22. [Available at https://doi.org/10.5479/si.00775630.66.1.]
-
Christophersen, E., 1931, Vascular plants of Johnston and Wake Islands: B.P. Bishop Museum Occasional Papers, v. 9, no. 13, p. 1–20.
-
Florida Invasive Species Council, 2019, 2019 FLEPPC list of invasive species: Florida Invasive Species Council online database, accessed November 12, 2019, at https://www.fleppc.org/.
-
Fosberg, F.R., 1959, Vegetation and flora of Wake Island: Atoll Research Bulletin, v. 67, p. 1–20. [Available at https://doi.org/10.5479/si.00775630.67.1.]
-
Fosberg, F.R., and Sachet, M.H., 1969, Wake Island vegetation and flora, 1961–1963: Atoll Research Bulletin, v. 123, p. 1–15. [Available at https://doi.org/10.5479/si.00775630.123.1.]
-
Gallagher, J.P., Grover, C.E., Rex, K., Moran, M., and Wendel, J.F., 2017, A new species of cotton from Wake Atoll, Gossypium stephensii (Malvaceae): Systematic Botany, v. 42, no. 1, p. 115–123, accessed October 10, 2017, at https://doi.org/10.1600/036364417X694593.]
-
Griffiths, R., Wegmann, A.S., Hanson, C., Keitt, B., Howald, G., Brown, D., Tershy, B., Pitt, W.C., Moran, M., Rex, K.R., White, S., Flint, B., and Torr, N., 2014, The Wake Island rodent eradication—Part success, part failure, but wholly instructive, in Vertebrate Pest Conference, 26th, Davis, Calif., 2014, Proceedings: Davis, Calif., University of California, Davis, p. 101–111.
-
Hathaway, S.A., Jacobi, J.D., Peck, R., and Fisher, R.N., 2022, Updates for Wake Atoll biosecurity management, biological control, survey, and management, and integrated pest management plans: U.S. Geological Survey Open-File Report 2022–1067, 56 p., accessed August 26, 2022 at https://doi.org/10.3133/ofr20221067.
-
Hawaiʻi Pacific Weed Risk Assessment, 2019, Hawaiʻi Pacific Weed Risk assessment 2019: Hawaiʻi Pacific Weed Risk Assessment, online database, accessed November 12, 2019, at www.hpwra.org.
-
International Union for Conservation of Nature (IUCN), 2019, Global invasive species database: Invasive Species Specialist Group (ISSG), Species Survival Commission (SSC), International Union for Conservation of Nature (IUCN), online database, accessed November 7, 2019, at http://www.iucngisd.org/gisd/.
-
Mueller-Dombois, D., and Fosberg, F.R., 1998, Vegetation of the tropical Pacific Islands: New York City, N.Y., Springer, 733 p. [Available at https://doi.org/10.1007/978-1-4419-8686-3.]
-
Roskov, Y., Abucay, L., Orrell, T., Nicolson, D., Bailly, N., Kirk, N., Bourgoin, T., DeWalt, R.E., Decock, W., DeWever, A., Nieukerken, E.V., Zarucchi, J., and Penev, L., 2019, Species 2000 and ITIS catalogue of life, 2019 annual checklist—Species 2000: Catalogue of Life online database, accessed March 4, 2019, at http://www.catalogueoflife.org/annual-checklist/2019/info/ac.
-
U.S. Department of Agriculture, 2019, Introduced, invasive, and noxious plants: U.S. Department of Agriculture, Natural Resources Conservation Services, online database, accessed November 4, 2019, at https://plants.sc.egov.usda.gov/java/.
-
U.S. Forest Service, 2018, Pacific Island ecosystems at risk (PIER), plant threats to Pacific ecosystems: U.S. Forest Service online database, accessed November 7, 2019, at http://www.hear.org/pier/.
Appendix B1. Plant Species Recorded from Wake Atoll in 2019
Available at https://doi.org/10.3133/ofr20231066.
Appendix B3. Plant Specimens Collected on Wake Atoll in 2019
Available at https://doi.org/10.3133/ofr20231066.
C. Introduction
The first systematic survey for terrestrial arthropods on Wake Atoll (hereafter Wake) was led by E.H. Bryan during the 1923 Tanager Expedition. From July 27 to August 5, 1923, Bryan and his colleagues collected and identified 44 species or morpho-species from within 11 arthropod orders (Bryan and others, 1926). After Bryan and others’ publication (1926), at least 23 notes and papers identifying additional specimens collected during the expedition, or possibly at other times, were published between 1928 and 1951 (publications listed in Bryan, 1959).
After the Tanager collection, relatively few additional arthropod species were recorded on Wake—notable among these was Bryan’s (1959) report that three species of mosquitoes were present during 1951: Culex quinquefasciatus, Aedes aegypti, and Aedes sp. (possibly A. scutellaris or A. albopictus). Because of their ability to vector pathogens harmful to humans, these mosquitoes became targets of an eradication attempt (Bryan, 1959). The effort primarily involved eliminating small reservoirs of water (for example, tires, cans, and other potential breeding habitats) and introducing western mosquitofish (Gambusia affinis) into larger bodies of fresh water, including ponds, bomb craters, and cisterns. Bryan (1959) reported that mosquitoes were not detected during his 2.5-day visit on the atoll in 1952, which indicates the eradication effort, or at least considerable control, seemed to have been successful.
Additional findings of arthropods on Wake Atoll include the identification of ectoparasitic mites (Laelaps nuttalli and Radfordia ensifera) and lice (Hoplopleura pacifica) on rats (Reeves and others, 2012) and the presence of the mango flower beetle (Protaetia fusca; Krell and Breidenbaugh, 2016). During 2009, a survey of terrestrial arthropods was done on Wake as part of an ecological monitoring program prior to an attempt to eradicate invasive rats from the atoll (Hebshi and others, 2011). In that study, at least 86 taxa were collected, 14 of which were identified to the species or near-species level. Subsequently, several arthropod taxa were identified during a survey for potential pollinators of plants being considered for propagation and habitat restoration (Center for Environmental Management—Military Lands, unpub. data, 2017). During the pollinator study, 7 species were detected visiting flowers, and 11 were identified from substrates other than flowers.
The objective of our work was to complete a broad survey of arthropods on Wake, which includes the three islets of Peale, Wake, and Wilkes, to provide a more complete understanding of the fauna, identify species that may pose a significant biosecurity risk, and create a photographic guide that can be used by resource managers and researchers as a tool to more easily identify arthropods that are encountered. This work also will provide a benchmark for comparison in future studies. To attain the goals of this project, our time and effort focused on sampling the atoll as extensively as possible rather than attempting to quantify the fauna within and among habitats or mapping distributions of individual species.
C. Methods
Arthropods were sampled from May 25 to June 8, 2019, using a suite of standard sampling methods, including pitfall traps, yellow pan traps, malaise traps, baiting for ants, light traps, litter sampling, yellow sticky card traps, mosquito traps, and collecting by hand (fig. C1). Each method targeted a slightly different sector of the fauna, but there was overlap in many instances. We attempted to survey as much of the atoll as possible, although the focus was on areas most affected by human activity (for example, dorms, operations facilities, storage areas, the airport, the boat harbor, the solid waste accumulation area, and ecologically complex habitats; fig. C2). We primarily surveyed outside habitats, but we also targeted the insides of several closed shipping containers. A brief description of each sampling method used during this study is listed here:
-
a. Malaise traps: Malaise traps are mesh tent-like structures that primarily intercept insects as they fly along the ground, but they also collect some ground-dwelling arthropods that may climb up onto vegetation. Bi-directional, Townes-style malaise traps fitted with a polyethylene collecting jar (BioQuip Products, Gardena, California) were used. Ethylene glycol mixture (antifreeze; a 50-percent solution with water) was used as a preservative in the collecting jars. Two malaise traps were deployed at seven locations representative of different habitats on Wake (five locations) and Peale (two locations) Islets. Once deployed, traps operated continuously and were moved to a new location about every 2–3 days.
-
b. Light traps: Lights operated at 9 are highly attractive to many adult Lepidoptera, Diptera, and Coleoptera. Battery-operated 25-watt ultraviolet lights were used to draw insects onto a white cotton sheet suspended vertically above the ground. Insects representing the different taxa encountered were collected by hand. A single light was operated for 1–2 hours after sunset at a variety of locations during 6 nights on Wake (5 nights) and Peale (1 night) Islets.
-
c. Pitfall traps: Pitfall traps primarily collect arthropods as they walk along the ground and can be effective at capturing spiders, Collembola, Isopods, and other flightless taxa. Traps consisted of 120 milliliter (mL) plastic specimen cups (5.7 centimeter [cm] diameter 7.2 cm deep) placed into the ground, so the top lip was even with the surface. Approximately 40 mL of an ethylene glycol mixture was placed into each cup to act as a preservative. A 27-cm diameter plastic picnic plate was suspended about 15 cm above each trap using rocks and sticks to prevent flooding during rain. Pitfall traps were placed in arrays of four to six traps at two locations on Wake. Each pitfall trap was allowed to operate for 3–4 days at each sample point.
-
d. Pan traps: Yellow-colored pan traps are designed to mimic flowers and foliage and are generally attractive to flying insects, such as Diptera and Hymenoptera but also collect arthropods attracted to water or that haphazardly fall into the traps. Traps were 15-cm diameter plastic picnic bowls that contained approximately 100 mL of water into which a drop of unscented liquid detergent was added to reduce surface tension and facilitate capture of insects. Pans were generally placed along transects at numerous locations (ranged from 4 to 14 pans per transect) under tree and shrub canopies on Peale, Wake, and Wilkes Islets. Pan traps were checked daily and operated for 1–2 days at each sample point.
-
e. Baiting for ants: Bait was used to attract and collect ants. Each bait consisted of approximately 5 gram of canned tuna mixed with a dollop of honey and placed on a 5×7 cm paper card and nestled within leaf litter. The baits were placed along the edges of buildings, shipping containers, or other structures. After about 60 minutes, ants were identified on the card, or the card containing the ants was collected. Baited sampling took place near the marina and around the shipping containers.
-
f. Litter sampling: Plant litter supports a variety of small, cryptic arthropods that are generally not collected using other methods. Litter was collected using a trowel and placed into 3.8 liter plastic Ziplock bags. In the dorm room, arthropods were separated from the litter using Berlese extractors powered by a 15-watt incandescent light bulb. Berlese extractors operate by creating a heat and moisture gradient that drives arthropods out of the litter (away from the light) and into a collecting vial placed at the bottom of the funnel. Litter was collected at six locations on Wake (four locations) and Peale (two locations) Islets. Extraction of arthropods from each litter sample took about 48 hours.
-
g. Yellow sticky cards: Yellow sticky cards (Seabright Laboratories, Emeryville, Calif.) are similar to pan traps in that they are designed to capture flying insects that are attracted to bright colors and can be effective at collecting parasitoid wasps and small flies. A clear sticky substance applied to the surface of the card captures insects upon contact. Yellow sticky cards were placed on inside and outside surfaces of several shipping containers and in the vegetation near the containers. Cards operated for 3–6 days at each sample point.
-
h. Mosquito traps: Center for Disease Control and Prevention (CDC) gravid mosquito traps (Model 1712; www.JohnWHock.com) were used to survey for mosquitoes. These traps consisted of a battery-operated fan-driven collection head placed over a 7.6 liter basin containing about 3.8 liter of “stinky water” that is attractive to female mosquitoes looking for egg-laying habitat. Stinky water was made by placing about 0.25 cubic meter of leaf litter (primarily ironwood litter) and several tablespoons of yeast starter (equal parts by weight of brewer’s yeast and lactalbumin) into 18.9 liter of water. This mixture was allowed to steep for at least 3 days before it was used in the mosquito traps. Traps were operated throughout the night at six locations near potential mosquito habitat (generally where there was a source of standing fresh water near buildings) on Wake Islet (near the dorms, mess hall, residential housing area, and main marina building).
-
i. Hand searching: Hand searching generally consists of shaking vegetation to dislodge arthropods onto a 70x70 cm nylon sheet supported by a wooden frame, sweeping vegetation with a net, overturning rocks and downed coarse wood debris, and excavating dead wood. Forceps or suction-driven aspirators were used to collect arthropods from the sheet, ground, or wood. Hand searching took place at numerous locations on Peale, Wake, and Wilkes Islets. Hand searching was used extensively for surveying for ants, particularly yellow crazy ants.
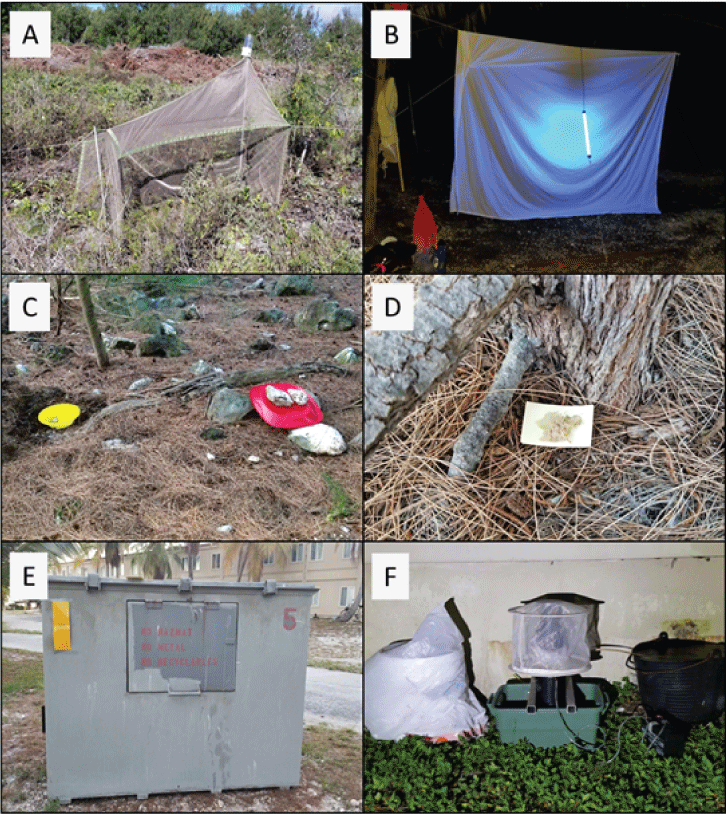
Methods used to collect arthropods, including malaise trapping: A, light trapping; B, pan trapping; C, (left) pitfall trapping, (right) under red rain cover; D, tuna baiting for ants; E, yellow sticky cards placed on dumpster; and F, (center) mosquito trapping. Methods used for extracting arthropods from leaf litter and for hand collecting are not shown. (Photographs by R. Peck, Hawaiʻi Cooperative Studies Unit, University of Hawaiʻi at Hilo, 2019).

Locations where the collection methods took place. Note that hand-searching, particularly for ants, took place over much of the atoll, and specific locations are not indicated. (WorldView 3 image, taken October 2015).
Most of the arthropods were identified by R. Peck by using existing keys, reference material in the entomology collection at the B.P. Bishop Museum, and online searches from reputable websites (for example, https://www.antweb.org). Neal Evenhuis (B.P. Bishop Museum) identified many of the Diptera, and Karl Magnacca (O'ahu Army Natural Resources Program) identified most of the Hymenoptera, except the ants. In several cases, it was not possible to confidently identify a specimen, or series of similar specimens, to the species level. In those cases, the species most closely thought to represent the specimen in question was preceded by an “nr.,” which indicates that it is near, or similar to, that species. In some other instances, specimens could only be identified to the genus, family, or in a few cases, order level.
For each taxon collected, we estimated the relative risk posed to base operations (for example, human health and operational effectiveness) or the ecology of the atoll and the feasibility of successfully managing the risk. Risk assessments were based on the expected effects for each species, whereas management feasibility was based on the likelihood of controlling the effect. These assessments were broadly categorized as low, medium, or high. A wide variety of published sources were used to estimate risks; the Invasive Species Specialist Group (ISSG) Global Invasive Species Database (GISD; International Union for Conservation of Nature, 2019) and Nishida and Evenhuis (2000) represent two of the more comprehensive sources. For many taxa, little or no information was available to guide the assessments. For these species, information from related species was used if available.
C. Results
Overall, 173 species or morpho-species from within at least 29 orders were identified during the survey (table C1; appendix C1). Hymenoptera (ants, bees, and wasps) were most diverse (33 species), followed by Coleoptera (beetles; 30 species), Diptera (flies; 25 species), and Araneae (spiders; 16 species). It was beyond the scope of this report to identify species and their abundances obtained using each collection method, but arthropods were generally collected as expected (see the “Methods” section). For example, flies and wasps were most commonly collected in malaise traps; spiders and true bugs were primarily obtained by collecting directly from vegetation; moths, termites, and some beetles and flies were collected using an ultraviolet light; many ants were attracted to baits; and mites, millipedes, and terrestrial spiders were extracted from litter using Berlese funnels.
To our knowledge, 81 arthropod species or morpho-species from 20 orders were previously documented on Wake (table C2). Most of those taxa appear to have been first collected during the Tanager Expedition (Bryan and others, 1926; Bryan, 1959), although 21 species were reported for the first time during the past 10 years. Overall, at least 17 of the previously documented species were collected during our survey, although it is possible that we detected an additional 16 taxa collected earlier; incomplete levels of identification (by us or by previous researchers) sometimes precluded species-level comparisons among surveys. Therefore, we report 140–156 arthropod species on Wake for the first time. In contrast, 48–64 species eluded us or are no longer present on the atoll.
Hebshi and others (2011) identified about 86 discrete arthropod taxa (in several additional cases it was unclear if taxa listed were different from those already listed), 15 of which were identified to species or near (listed as “nr.”) species level. Of those taxa, we collected as many as nine species. Unfortunately, we were not able to locate the collection described in Hebshi and others (2011; Bishop Museum Accession Number 2010.010), so we were unable to identify their undetermined taxa or compare our specimens to those in their collection.
Shipping containers are a significant biosecurity concern because arthropods that can survive conditions within containers are easily transported between locations. In two accessible containers that housed pallets, cardboard, or other material (fig. C3), we detected one or more of the following arthropods: orange-flanged millipede (Polydesmida: Paradoxosomatidae), juvenile cockroach (Blattodea), silverfish (Zygentoma: Lepismatidae), pholcid spider (Araneae: Pholcidae), isopod (Isopoda), springtail (Collembola: Entomobryidae), pygmy stinkbug (Hemiptera: Cydnidae), and several ant species, including the Singapore ant, the robust crazy ant, and the ghost ant. Each species collected tolerates dark, enclosed locations (for example, pholcids), particularly where moisture may persist, like under cardboard or wooden boards. Holes in the containers may allow some taxa to move freely to the outside, although pholcids, isopods and cockroaches may reside fully within the containers. A robust crazy ant nest (including a queen and her brood) was detected under a cardboard box in one container. Three small flies (not identified) were caught on a yellow sticky card placed in one container. Insects were not collected on a yellow sticky card placed in a second container.

A, Shipping containers lined up in the industrial area on Wake Islet; and B, Debris in the shipping containers (provides habitat for some arthropods). (Photographs by R. Peck, 2019, Hawaiʻi Cooperative Studies Unit, University of Hawaiʻi at Hilo).
C. Discussion
In natural conditions, the arthropod community detected on a remote, low-lying atoll like Wake would be expected to be relatively depauperate due to the low diversity of habitats and plant species richness. However, arthropod species diversity may increase with human colonization as additional plant species are introduced and the habitat is modified in ways that benefit species that thrive in proximity to humans. During our survey of Wake, we collected about 170 species across much of the three islets that comprise the 655-hectare (ha) atoll, revealing a relatively rich arthropod fauna. For comparison, 115 arthropods were identified on Palmyra Atoll (less than 500 ha; Handler and others, 2007), and about 73 species were identified on Rose Atoll (6.6 ha; Peck and others 2014). In contrast, we detected considerably fewer species than observed on Midway Atoll, which is similar in size to Wake Atoll (627 ha; Nishida and Beardsley, 2002). On Midway, 546 species were reported from 1891 to 1999. The number of arthropods identified on Midway increased dramatically during the past century because only 38 species were collected during the Tanager Expedition (Bryan and others, 1926). By 1960, the number of species identified had increased to 221 (Suehiro, 1960), and 331 species were collected during 1997–99 (Nishida and Beardsley, 2002). The tremendous increase in arthropod species richness over time on Midway was largely attributed to a parallel increase in the diversity of plants that led to an increase in available niches.
It is not surprising that we failed to collect numerous arthropod species that had been reported previously from Wake. Many species can be expected to be uncommon, temporally variable in abundance, or very patchily distributed, making them difficult to collect during a brief survey. For several species, we collected only one individual, indicating that more species could have been identified with additional effort. Similarly, we detected additional species on the day we departed the atoll (at the airport terminal), indicating that more work could have resulted in more species being detected. It also is possible that some species detected by earlier researchers no longer exist on Wake. It is likely that some species became extirpated over time through depredation, competitive displacement, changes in habitat quality, or by other mechanisms. Small islands and islets, such as those that make up Wake Atoll, are particularly susceptible to stochastic events (for example, storms and drought) that sometimes have significant effects on small populations.
Many of the arthropods collected during our survey pose a low level of risk to the biosecurity of Wake Atoll, but several species pose medium to high levels of risk. One important group is ants, which is a suite of species that have increased greatly in diversity since the Tanager Expedition. Most of the ant species we collected are considered tramp species (strongly associated with humans) and are widespread across the Pacific Basin. Overall, we collected 14 ant species that were not reported by Bryan and others (1926). The yellow crazy ant (Anoplolepis gracilipes), big-headed ants (three species of Pheidole), longhorn crazy ant (Paratrechina longicornis), and tropical fire ant (Solenopsis geminata) are significant because they pose considerable risk to the biodiversity of Wake (O’Dowd and others, 2003; Wetterer, 2007; Wetterer, 2008; Wetterer, 2011). The habitation of the yellow crazy ant on the atoll is of particular concern because it has been indicated to have detrimental effects on ground-nesting seabirds (Plentovich and others, 2009; Plentovich and others, 2018). We detected three of the four ant species collected by Bryan and others (1926) during our survey.
A species that is considered to pose a medium-high level of risk to the biodiversity of Wake is the urbicola scale (Pulvinaria urbicola). This herbivorous insect was detected on Pisonia grandis trees and may affect the health of this tree species on the atoll. The urbicola soft scale feeds on leaves and is capable of stressing trees until they eventually die. Many Pisonia stands across the Pacific and Indian Oceans have been negatively affected by the urbicola soft scale insect (Handler and others, 2007; Neumann and others, 2014).
It is difficult to determine the provenance of many terrestrial arthropods on oceanic islands. Although Wake surely supported a native community of insects and other arthropods, generally poor knowledge of native home ranges for most species hinders our ability to know which species are native to the atoll. In many cases, it is easier to determine which species are not native than which species are native. Bryan and others (1926) reported that many of the arthropods that they encountered were detected elsewhere in the South Pacific. Even at that early time, it is likely that many species had been transported to Wake by humans. For example, the three species of ants that Bryan and others identified on Wake are known to be tramp species strongly associated with human movement across the Pacific Basin. Although it was beyond the scope of this survey to identify the arthropods associated with each of the many plant species, it is notable that we collected several Acalles wilkesii on the endemic cotton (Gossypium stephensii). This species also was collected by Bryan and others (1926), and Wake seems to represent the northernmost extent of this Pacific-wide genus of weevils (Zimmerman, 1938). A thorough search of the literature may lead to confident determination of provenance for some species, but that is beyond the scope of our study.
Table C1.
List and status of arthropods collected during the 2019 survey of Wake Atoll.[Function: U, unknown; D, detritivore; F, fungivore; Pa, parasite; Pr, predator; S, scavenger; O, omnivore; H, herbivore; B, blood feeder; K, kleptoparasite; PS, parasitoid; Po, pollen feeder. Status: U, unknown; A, alien; I, invasive; N, native; NA, not applicable/native species. Impact type: U, unknown; HH, human health; S, structural damage; A, agricultural pest; B, biodiversity; FS, food storage pest; NA, not applicable/native species. Islet detected: P, Peale; Wa, Wake; Wi-N, Wilkes (north islet); Wi-S, Wilkes (south islet). Risk assessment: H, high; M, medium; L, low; NA, not applicable/native species. Management feasibility: H, high; L, low; M, medium; NA, not applicable/native species]
Table C2.
List of arthropod species collected at Wake Atoll prior to 2019.[This list was compiled from various sources, including unpublished data. Full citations for published documents can be found in chapter C, section "References Cited"]
C. References Cited
-
Aldrich, J.M., 1931, New acalyptrate Diptera from the Pacific and Oriental regions: Proceedings of the Hawaiian Entomological Society, v. 7, no. 3, p. 395–399.
-
Bryan, E.H., Jr., 1926, Insects of the Tanager Expedition [abs.], in Hawaiian Academy of Science Conference, 1st, Honolulu, Hawaii, 1926, Proceedings: Honolulu, Hawaii, Hawaiian Academy of Science, Bishop Museum Special Publication 11, 31 p.
-
Bryan, E.H., Jr., 1948, Flies on Wake, in Hawaiian Entomological Society Conference, 13th, Honolulu, Hawaii, 1948, Proceedings: Honolulu, Hawaii, Hawaiian Entomological Society, v. 13, 221 p.
-
Bryan, E.H., Jr., 1959, Notes on the geography and natural history of Wake Island: Atoll Research Bulletin, no. 66, p. 1–22. [Available at https://doi.org/10.5479/si.00775630.66.1.]
-
Bryan, E.H., Jr., Timberlake, P.H., Wheeler, W.M., Van Zwaluwenburg, R.H., Perkins, R.C.L., Shannon, R.C., Swezey, O.H., Hebard, M., and Chamberlin, R.V., 1926, Insects of Hawaii, Johnston Island and Wake Island: Bernice P. Bishop Museum Bulletin 31, Tanager Expedition Publication, no. 3, p. 1–94.
-
Chilson, L.M., 1953, Fleas on Wake Island, in Hawaiian Entomological Society, Proceedings: Honolulu, Hawaii, Hawaiian Entomological Society, v. 15, no. 1, 1 p.
-
Gross, G.F., 1963, Insects of Micronesia—Coreidae (Alydini by J.C. Schaffner), Neididae, and Nabidae: Honolulu, Hawaii, Bernice P. Bishop Museum, v. 7, p. 357–390. [Available at http://hbs.bishopmuseum.org/pubs-online/pdf/iom7-7cor.pdf.]
-
Handler, A.T., Gruner, D.S., Haines, W.P., Lange, M.W., and Kaneshiro, K.Y., 2007, Arthropod surveys on Palmyra Atoll, Line Islands, and insights into the decline of the native tree Pisonia grandis (Nyctaginaceae): Pacific Science, v. 61, no. 4, p. 485–502. [Available at https://doi.org/10.2984/1534-6188(2007)61[485:ASOPAL]2.0.CO;2.]
-
Hebshi, A., Kesler, D., and Zabin, C., 2011, Ecological monitoring on Wake Island prior to rat removal: Department of Defense Legacy Resource Management Program, Project 09–438, 84 p., accessed April 15, 2019, at https://denix.osd.mil/nr/otherconservationtopics/installationspecificinformation/wake-atoll/ecological-monitoring-compendium-on-wake-island-prior-to-r at-removal-final-technical-report-november-2011-legacy-09-438/.
-
Hull, F.M., 1937, A check list of the Syrphidae of Oceania: Honolulu, Hawaii, Bishop Museum Occasional Papers, v. 13, no. 10, p. 79–87.
-
International Union for Conservation of Nature, 2019, Global invasive species database: Invasive Species Specialist Group (ISSG), Species Survival Commission (SSC), International Union for Conservation of Nature (IUCN), online database, accessed November 7, 2019, at http://www.iucngisd.org/gisd/.
-
Jacot, A.P., 1928, New oribatoid mites: Psyche—A Journal of Entomology, v. 35, p. 213–215. [Available at https://doi.org/10.1155/1928/51346.]
-
Jacot, A.P., 1929, Concerning the genus Neolides (Oribatoidea-Acarina): Transactions of the American Microscopical Society, v. 48, no. 1, p. 30–48. [Available at https://doi.org/10.2307/3222456.]
-
Jacot, A.P., 1934, Some Hawaiian Oribatoidea (Acarina): Honolulu, Hawaii, Bishop Museum Bulletin, v. 121, p. 1–99.
-
Krell, F.T., and Breidenbaugh, M., 2016, The mango flower beetle, Protaetia fusca (Herbst), on Wake Island, Western Pacific Ocean (Coleoptera—Scarabaeidae—Cetoniinae)—An accomplished island invasive: Proceedings of the Hawaiian Entomological Society, v. 48, p. 9–13.
-
Lieftinck, M.A. 1962, Insects of Micronesia—Odonata: Honolulu, Hawaii, Bernice P. Bishop Museum, Insects Micronesia v. 5, p. 1–95. [Available at http://hbs.bishopmuseum.org/pubs-online/pdf/iom5-1.pdf.]
-
Neumann, G., O’Dowd, P.T., Gullan, P.J., and Green, P.T., 2014, First record of Pulvinaria urbicola Cockerell (Hemiptera—Coccidae), a potentially damaging scale insect, on Christmas Island, Indian Ocean: Journal of Asia-Pacific Entomology, v. 17, no. 1, p. 27–30. [Available at https://doi.org/10.1016/j.aspen.2013.09.001.]
-
Nishida, G.M., and Beardsley, J.W., 2002, A review of the insects and related arthropods of Midway Atoll: Bishop Museum Occasional Paper, no. 68, p. 25–69.
-
Nishida, G.M., and Evenhuis, N.L., 2000, Arthropod pests of conservation significance in the Pacific—A preliminary assessment of selected groups, in Sherley, G., ed., Invasive species in the Pacific—A technical review and draft regional strategy—South Pacific Regional Environment Programme: Samoa, SPREP, p. 115–142.
-
O’Dowd, D.J., Green, P.T., and Lake, P.S., 2003, Invasional ‘meltdown’ on an oceanic island: Ecology Letters, v. 6, no. 9, p. 812–817. [Available at https://doi.org/10.1046/j.1461-0248.2003.00512.x.]
-
Peck, R., Banko, P., Pendleton, F., Schmaedick, M., and Ernsberger, K., 2014, Arthropods of Rose Atoll with special reference to ants and Pulvinaria urbicola scales (Hemiptera: Coccidae) on Pisonia grandis trees: Hilo, Hawaii, Hawaii Cooperative Studies Unit Technical Report HCSU-057, Hawaiʻi Cooperative Studies Unit, University of Hawaiʻi.
-
Plentovich, S., Hebshi, A., and Conant, S., 2009, Detrimental effects of two widespread invasive ant species on weight and survival of colonial nesting seabirds in the Hawaiian Islands: Biological Invasions, v. 11, p. 289–298. [Available at https://doi.org/10.1007/s10530-008-9233-2.]
-
Plentovich, S., Russell, T., and Fejeran, C.C., 2018, Yellow crazy ants (Anoplolepis gracilipes) reduce numbers and impede development of a burrow-nesting seabird: Biological Invasions, v. 20, p. 77–86. [Available at https://doi.org/10.1007/s10530-017-1516-z.]
-
Reeves, W.C., 1953, Possible recent introductions of mosquito vectors of human diseases in the central Pacific: Proceedings of the 7th Pacific Science Congress, v. 7, p. 371–373.
-
Reeves, W.K., Utter, C.M., and Durden, L., 2012, Rickettsial pathogens and arthropod vectors of medical and veterinary significance on Kwajalein Atoll and Wake Atoll: Micronesica, v. 43, no. 1, p. 107–113.
-
Rosen, L., Reeves, W.C., and Aarons, T., 1948, Aedes aegypti on Wake, in Hawaiian Entomological Society Conference, 13th, Honolulu, Hawaii, 1948, Proceedings: Honolulu, Hawaii, Hawaiian Entomological Society, v. 13, p. 255–256.
-
Suehiro, A., 1960, Insects and other arthropods from Midway Atoll: Proceedings of the Hawaiian Entomological Society, v. 17, p. 289–298.
-
Usinger, R.L., 1937, Two new Pacific Island species of Nysius (Lygaeidae-Hemiptera), Proceedings: Honolulu, Hawaii, Hawaiian Entomological Society, v. 9, no. 3, p. 439–442.
-
Usinger, R.L., 1941, The genus Oechalis (Pentatomidae, Hemiptera), Proceedings: Honolulu, Hawaii, Hawaiian Entomological Society, v. 11, no. 1, p. 59–93.
-
Van Zwaluwenburg, R.H., 1948, New species and new records of elaterid beetles from the Pacific, III, in Hawaiian Entomological Society Conference, 13th, Honolulu, Hawaii, 1948, Proceedings: Honolulu, Hawaii, Hawaiian Entomological Society, v. 13, p. 265–276.
-
Wetterer, J.K., 2007, Biology and impacts of Pacific Island invasive species. 3–The African Big-headed ant, Pheidole megacephala (Hymenopotera—Formicidae): Pacific Science, v. 61, no. 4, p. 437–456. [Available at https://doi.org/10.2984/1534-6188(2007)61[437:BAIOPI]2.0.CO;2.]
-
Wetterer, J.K., 2008, Worldwide spread of the longhorn crazy ant, Paratrechina longicornis (Hymenoptera—Formicidae): Myrmecological News, v. 11, p. 137–149.
-
Wetterer, J.K., 2011, Worldwide spread of the tropical fire ant, Solenopsis geminata (Hymenoptera—Formicidae): Myrmecological News, v. 14, p. 21–35.
-
Williams, F.X., 1945, Achaea janata (Linn.), in Hawaiian Entomological Society Conference, Proceedings: Honolulu, Hawaii, Hawaiian Entomological Society, v. 12, 233 p.
-
Zimmerman, E.C., 1938, The status of Acalles wilkesii (Coleoptera, Curculionidae): Proceedings of the Hawaiian Entomological Society, v. 10, no. 1, p. 150–151.
Chapter D. Wake Atoll 2019 Terrestrial Reptile Species Survey Report and Field Guide
By Stacie A. Hathaway, Adam R. Backlin, Cynthia J. Hitchcock, and Robert N. Fisher
D. Introduction
Historically, amphibians have not been reported, but reptiles have been observed at Wake during various expeditions, and voucher specimens have been deposited in museum collections since 1871. These collections and observations have been largely opportunistic and referenced in various published and unpublished literature (for example, Bryan, 1949; Bryan, 1959; Ineich and Zug, 1991; Crombie and Pregill, 1999; Ogden Environmental and Energy Services, Co., Inc., unpub. data, 1999; U.S. Air Force, unpub. data, 2017). Typical of small, remote, islands and atolls, the terrestrial reptile community has been presumed to consist of very few species. Before this study, the Wake terrestrial reptile community had never been formally surveyed. It is important to fill in knowledge gaps regarding the biodiversity at Wake and to establish a current (2019) baseline for evaluating change over time. This effort is essential in understanding the processes that give rise to Wake’s biodiversity and the status of processes that may threaten species. Nonnative herpetofauna (amphibians and reptiles) may pose a threat directly or indirectly to other taxa, natural biological processes, or even human infrastructure, and therefore, knowing their source and distribution is critical for making management decisions. These surveys serve as a basis for examining the status and distribution of reptile species known from Wake historically and can be used as a general assessment of the success of recent (2012) biosecurity measures in preventing new species arrivals and in identifying improvements to consider for the protocols currently (2019) in place.
In spring 2019 (between May 24 and June 7), we surveyed during a 2-week field visit as one component of a larger project to update and assess the status of terrestrial plant, arthropod, and reptile communities on Wake. Rapid assessment surveys for reptiles were done with several objectives: (1) compile an updated species list to determine presence, and to the extent possible, the status and distribution of historically known species; (2) determine the presence of any new potentially invasive reptile species and any other invasive species with known or presumed effects on reptiles or other taxa native to Wake, with an emphasis on the yellow crazy ant (YCA) Anoplolepis gracilipes; (3) create a Wake reptile photograph identification guide; and (4) use these data to inform biosecurity and integrated pest management plans for Wake (Hathaway and others, 2022).
D. Methods
We compiled Wake Atoll-specific data from museums, as well as from published and unpublished literature, and interviewed people with local knowledge to summarize the status and distribution of reptiles known historically to exist on the atoll.
As an initial step in an effort to inform the creation of long-term management strategies for invasive and other pest species at Wake, we carried out a small-scale internal preliminary risk analysis of species detected on Wake (Hathaway and others, 2022). We assessed introduced species as a potential threat to natural resources or base operations (for example, human health and the overall installation mission) on Wake if they were established and invasive elsewhere. Risk ranges were categorized from high to low based on published risk assessments or observations elsewhere while also considering conditions on Wake. We reviewed a variety of published sources, such as the Invasive Species Specialist Group (ISSG) Global Invasive Species Database (GISD; International Union for Conservation of Nature, 2019) to assess risk and management feasibility. Management feasibility, the likelihood of successful eradication or control, was categorized from very low to very high, with consideration given to several criteria, such as potential for effectiveness, practicality, cost, potential negative effects from control or eradication, and likelihood of reinvasion. The feasibility of controlling a species varies depending on the actual distribution of the species and the availability of effective control tools or protocols. We include the status and preliminary risk results for species detected on Wake in table D1 and for species included in the “Field Guide” section of this chapter.
We completed reptile surveys broadly across Wake, including all three islets. Wake, Wilkes, and Peale Islets have been separated into a series of habitat management units (HMUs) delineated to assist in the creation of natural resources management actions and approaches (U.S. Air Force, unpub. data, 2017). We used the HMUs to reference specific focus areas we considered most likely to be vulnerable to invasive species incursions (for example, the marina and associated areas within HMU-11, areas with concentrated human populated buildings, such as in HMU-58, and cargo-container unloading and storage areas within HMU-65; appendix D1, fig. D1.1).
Field Surveys
Herpetofauna surveys included three types of sampling to collect the most data on a suite of species with differing habits in a short amount of time. These methods were sticky glue-board trap transects, daytime visual encounter surveys (VES), and nighttime VES in dry weather conditions. Trap transects were composed of a series of individual glue boards measuring 19 centimeters (cm) by about 14 cm (fig. D1). These glue boards are the type used to ensnare rodents and insects and can passively capture lizards as they walk over the adhesive surface (for example, Trapper Max Free Mouse Glue Traps). Ensnared individuals were removed by applying cooking oil (for example, palm or vegetable), which degrades the adhesive from the parts of the body in contact with the board. All survey methods were employed on each of the three islets (Wake, Peale, and Wilkes).

Example of a sticky glue board attached to a tree. (Photograph by A.R. Backlin, U.S. Geological Survey, 2019).
Glue-board trap transects ranged from 0.25 to 1 kilometer (km) long, and trap stations were set 5–100 meters (m) apart and were not restricted to individual habitat types. Each station consisted of up to three glue boards, with one board placed on the ground at each station, and if there is a log, we place a trap on a log, and if there is a tree, we place a trap on a tree 1 m off the ground. This configuration was intended to capture lizard species with different behavioral tendencies. We recorded latitude and longitude coordinates of each trap station. Initially, glue boards were checked frequently to determine how long they could be left out, which was determined by shade available, heat, which effects duration of “stickiness,” and captures of or disturbance by non-target species. Glue boards on transects were left out up to 24 hours, conditions permitting. Reptiles were identified to species, removed and, depending on number of captures, up to 20 individuals of each species were weighed and measured (ventrally from tip of nose to cloaca; snout to vent length). Up to 10 of each species per transect were collected as voucher specimens following the general protocol by McDiarmid (1994). After euthanizing, weighing, and measuring, we removed a tissue sample for deoxyribonucleic acid (DNA), and the specimen was pickled in a 10-percent buffered formalin solution. As many lizards as possible were tail clipped for DNA. All tissue samples for DNA were stored in 95-percent ethanol. Multiple transects were run on Wake, Peale, and Wilkes Islets.
Daytime and nighttime visual encounter surveys were done (mostly) by walking slowly through the different habitat types following the glue-board transects, although some surveys were longer than those transects, and additional searches were made around buildings. We scanned ahead, on the ground and in trees, while occasionally disarticulating fallen logs, peeling bark off trees, looking in root systems, going through litter, and turning over logs and rocks. Each lizard seen was counted and identified to species if possible. We attempted to hand-capture animals when possible and when necessary for identification. Habitat information was also noted. Daytime and nighttime surveys were done during non-rain events. Due to time constraints, daytime visual encounter surveys were generally concentrated on searches for novel species—any species not detected on glue boards or during nighttime visual encounter surveys. This process included any potential incidental observations of nonnative taxa, particularly amphibians or arthropods.
Glue boards also provided a method for detecting arthropods. We focused on documenting the distribution of the invasive YCA because of its adverse ecological effects on other Pacific Islands. This species has been introduced into numerous tropical and subtropical islands, including those in the Caribbean, the Indian Ocean (Seychelles, Madagascar, Mauritius, Réunion, the Cocos Islands, and the Christmas Islands), and in the Pacific (New Caledonia, Hawai`i, French Polynesia, Okinawa, Vanuatu, Micronesia, and the Galapagos archipelago; McGlynn, 1999; Holway and others, 2002; Wetterer, 2005). When collecting traps, we examined them for any arthropods we had not seen on previously collected traps and kept those traps for our arthropod expert (Robert Peck) for identification (see chapter C “Wake Atoll 2019 Arthropod Species Survey Report and Field Guide”) to supplement his surveys. We also hand-collected a few macro-invertebrates.
D. Results
Historical Records
Our initial review of museum records indicated eight terrestrial reptile species had been collected from Wake, with the first reptile record, the Oceania snake-eyed skink (Cryptoblepharus poecilopleurus), collected in 1871 (fig. D2; table D1). We identified at least two misidentifications in the records. We verified eight species deposited in museums: the Oceania snake-eyed skink, azure-tailed skink (Emoia cyanura), dark-bellied copper-striped skink (Emoia impar), mourning gecko (Lepidodactylus lugubris), common house gecko (Hemidactylus frenatus), stump-toed gecko (Gehyra insulensis), Brahminy blindsnake, (Ramphotyphlops braminus), and the gopher snake (Pituophis catenifer; figs. D2–D11; table D1).

Oceania snake-eyed skink (Cryptoblepharus poecilopleurus) from Wake Atoll. (Photographs by A.R. Backlin, U.S. Geological Survey, 2019).

Azure-tailed skink (Emoia cyanura) from Wake Atoll. (Photograph by A.R. Backlin, U.S. Geological Survey, 2019).

Azure-tailed skink (Emoia cyanura), melanistic phenotype from Wake Atoll. (Photograph by A.R. Backlin, U.S. Geological Survey, 2019).

Dark-bellied copper-striped skink (Emoia impar; photograph by C.W. Brown, U.S. Geological Survey, 2006).

Mourning gecko (Lepidodactylus lugubris) from Wake Atoll. (Photograph by A.R. Backlin, U.S. Geological Survey, 2019).

Common house gecko (Hemidactylus frenatus) from Wake Atoll. (Photograph by A.R. Backlin, U.S. Geological Survey, 2019).

Pacific stump-toed gecko (Gehyra insulensis). (Photograph by A.R. Backlin, U.S. Geological Survey, 2009).

Brahminy blindsnake (Ramphotyphlops braminus) from Wake Atoll. (Photograph by J. Gilardi, Island Conservation, 2017).
There was a misidentification in the museum records, which included four specimens identified as the metallic skink (Lygosoma metallicum). Upon review of the specimens, we realized they were Oceania snake-eyed skinks (fig. D11). Bryan (1959) reported that a brown tree snake (Boiga irregularis) was collected at Wake in 1948, and that finding has been circulating in the literature since then. The specimen identification was verified and had been updated in the museum records as a gopher snake in 1987; however, it continued to be referenced in the literature as the brown tree snake. We procured photographs of the specimen and include it here for species verification (fig. D10).

Gopher snake (Pituophis catenifer) specimen from Wake Atoll in1948 (previously identified as a brown tree snake [Boiga irregularis]). (Photograph by B.P. Bishop Museum, 2019).

Oceania snake-eyed skink (Cryptoblepharus poecilopleurus) specimen from Wake Atoll identified in the museum record as a metallic skink (Lygosoma metallicum). (Photographs by Brigham Young University Life Science Museum, 2019).
While reviewing unpublished literature, we found reference to an additional nonnative species mentioned for Wake. A worker at Wake, possibly a member of the TEC Inc. staff, reported seeing a green anole (Anolis carolinensis) during a 2008 site visit to Wake (U.S. Air Force, unpub. data, 2017). Additional information was not provided, and other documentation supporting this observation was not located.
Field Surveys
We captured 206 lizards, detecting 4 of the 8 verified species of reptiles previously recorded at Wake on glue-board traps set across the 3 islets (178 trap stations with 458 total glue boards; table D2). We were able to place at least 1 glue-board trap station within 27 of the 65 habitat mapping units delineated for natural resource management of Wake (table D2; appendix D1, fig. D1.1). These sites represented most of the dominant types of habitat present. Most glue boards were open for some period during daytime and nighttime hours for roughly equivalent durations (ranged from 100 to 591 minutes open in daylight and 80 to 300 minutes at night). Because of time, logistics, and safety, some transects were only open during the daytime (that is, Peale Coast) or nighttime (that is, Peale North and the mowed area of the Bird Sanctuary). One trap station was not re-opened at night due to proximity to wedge-tailed shearwaters. Table D2 provides information about the HMUs surveyed, number of transects and traps, and species captured for each islet. We detected YCAs on glue boards broadly across the atoll on parts of all three islets; YCAs were not detected on Wilkes North, which complemented what was detected during arthropod surveys (see chapter C; table D2; appendix D1, figs. D1.2, D1.3). A variety of arthropods were given to the arthropod expert (R. Peck) for identification and for including in the results of the arthropod surveys (chapter C). We also observed high numbers of the Pacific rat (Rattus exulans) throughout Wake Islet and Wilkes Islet; Pacific rats were not detected on Peale. We did not detect any amphibians.
We detected 417 lizards across all nighttime visual encounter surveys. Of these lizards, 76 percent were common house gecko detections compared to 23 percent mourning geckos, and 1 percent azure-tailed skinks (table D3). During daytime visual encounter surveys, we did not encounter any species that had not been recorded on glue boards or during nighttime visual encounter surveys. However, we did encounter and capture three additional Oceania snake-eyed skinks in a new location, near the visitor lodging in HMU 58 the day before leaving Wake.
We collected 62 individuals for museum vouchers and an additional 61 tail tips across the 4 species encountered (table D4; appendix D2, table D2.1). A single melanistic azure-tailed skink species was detected on Wake Islet, and all four species were collected on each of the three islets although not on every transect (table D2; appendix D1, figs. D1.2, D1.4–D1.7). Based on our surveys, the Oceania snake-eyed skink, azure-tailed skink, mourning gecko, and common house gecko can be considered established and generally widespread (appendix D1, figs. D1.2, D1.4–D1.7; table D2).
Summary of Reptile Species
For additional description, see the “Field Guide to the Herpetofauna of Wake Atoll” section (appendix D3); locations of reptile detections are noted in appendix D1.
Skinks
The Oceania snake-eyed skink (Cryptoblepharus poecilopleurus; tables D1, D2, D4; fig. D2; appendices D1 [figs. D1.2, D1.4] and D3) is native to Wake. There were relatively low detections of this species, with eight (4 percent) captures on less than 2 percent of the glue boards across three transects and five (19 percent) of the HMUs surveyed. The Oceania snake-eyed skink was captured on all three islets, mostly on stations in open vegetation nearest the coast. This species was originally collected as a single record in 1871 and was collected from all three islets in 1923.
The azure-tailed skinks (Emoia cyanura; tables D1–D4; figs. D3, D4; appendices D1 [figs. D1.2, D1.5], D2) may be native to Wake or could have been accidentally introduced before World War II (WWII). The first records of this species were from the Tanager Expedition in 1923. Azure-tailed skinks were the most detected and widespread species captured using glue boards. The azure-tailed skink was detected with 74 percent of all captures on 21 percent of all glue boards across 11 transects and 20 (74 percent) of the HMUs surveyed. This species was detected almost equally on boards, the ground, and on logs in almost every habitat type. We detected a single capture of a melanistic azure-tailed skink on a glue board (table D2; fig. D4) on a log under Terminalia catappa. This detection was in open native vegetation along a road in HMU 27. This record is the first of a melanistic azure-tailed skink at Wake. Melanism can be common in some far eastern populations of this species, such as Clipperton Atoll, and these can be difficult to identify from melanistic dark-bellied copper-striped skinks (Bruna and others, 1996a, 1996b). This species is known to tolerate even extreme disturbance (Bruna and others, 1996a).
The dark-bellied copper-striped skink (Emoia impar; table D1; fig. D5; appendix D3) may be native to Wake or could have been accidentally introduced before WWII. The only record of this species is a single individual collected as part of the Tanager Expedition in 1923. We did not detect this species during our survey.
Geckos
The mourning gecko (Lepidodactylus lugubris; tables D1–D4; fig. D6; appendices D1 [figs. D1.2, D1.6] and D3) is considered native to Wake and was first recorded on Peale Islet by the Tanager Expedition in 1923. Mourning geckos accounted for 7 percent of all lizard captures on glue boards. We detected mourning geckos on 63 percent of our nighttime searches. There were about equivalent numbers of common house geckos and mourning geckos on 4 of the 10 surveys, during which both species were detected; otherwise, there were considerably higher numbers of common house geckos on all other surveys (table D3). We consider mourning geckos native because the presumptive area of origin for this hybrid parthenogenic species is the southern Marshall Islands at or near Arno Atoll (Radtkey and others, 1995). Mourning geckos have clearly spread with humans more recently, and Ineich (1999) reported high clone turnover in some places due to the movement of people and equipment during WWII. For Wake, this species could have arrived on its own with early Micronesians and Europeans. Mourning geckos have probably arrived several times and could have experienced this clonal turnover. Because we cannot resolve this question, we conservatively consider mourning geckos are native.
The common house gecko (Hemidactylus frenatus; tables D1–D4; fig. D7; appendices D1 [figs. D1.2, D1.7] and D3) is not native to Wake and is considered invasive. The common house gecko was the second most detected species on glue boards, with 32 (16 percent) captures on 9 percent of all glue boards across 8 transects and 13 (48 percent) of the HMUs surveyed. We detected common house geckos on 100 percent of our nighttime visual encounter surveys (table D3). We detected common house geckos in great abundance on lighted and dark structures, rock walls, and on vegetation, including Heliotropium and Casuarina. Common house geckos seemed especially numerous on the dead or dying trees with peeling bark. We detected one common house gecko in the Lepturus field on Peale Islet. Common house geckos were first collected at Wake in 1957.
The Pacific stump-toed gecko (Gehyra insulensis; table D1; fig. D8; appendix D3) is considered a pre-WWII invasive species to Wake and was first recorded by the Tanager Expedition in 1923. The Pacific stump-toed gecko is considered a relatively recent invader in the Pacific during the last few hundred years because of European traders (Fisher, 1997). This species has been seen to decline over time in places that are invaded by the common house gecko, and we did not detect it on Wake during our survey.
Snakes
Brahminy blindsnakes (Ramphotyphlops braminus; table D1; fig. D9; appendix D3) are not native to Wake. We did not see any Brahminy blindsnakes during these surveys; however, we received photograph documentation of this species on Peale Islet in 2017 from John Gilardi of Island Conservation (fig. D9). The original museum record had been collected on Wake Islet in 1998. The Brahminy blindsnake is potentially localized on Wake and Peale; however, this cryptic fossorial species is mostly nocturnal and generally detected by moving soil, leaf litter, rocks and debris, or occasionally on the surface during rains. The species resembles an earthworm, so it is often overlooked.
Invertebrates
We detected YCAs on 137 of 458 (or 30 percent) of all glue boards and YCA detections on 98 percent of the glue boards used on Peale (table D2). We did not find any YCAs at the Bird Sanctuary (HMUs 1–3) on Wilkes. We also observed another invertebrate species of concern, the Asian forest centipede (Scolopendra subspinipes), on Peale.
D. Discussion
This work is the first formal reptile survey at Wake and compiles what is known regarding historic and current (2019) status and distribution, providing information that can be used to modify and create more management measures as well as evaluate their current (2019) and future success. As expected, and typical of remote, isolated, small islands and atolls, the Wake terrestrial reptile species community consists of very few species. During these surveys, we detected three species presumed to be native, two skinks and one gecko, as well as one nonnative gecko species. It is important that we detected the Oceania snake-eyed skink for which there had been previous concern regarding its possible extirpation. Given that recent reviews of the genus (Horner, 2007; Blom and others, 2019) continue to reveal previously undescribed species, future genetic and morphologic analyses of the specimens from Wake could show it to be endemic. Besides Wake, the closest location where this species is known is Bokak Atoll in the Marshall Islands (Buden and others, 2020). The Oceania snake-eyed skink is absent from the rest of the Marshall Islands and all the other Caroline Islands (Crombie and Pregill, 1999; Buden and Taboroši, 2015). The other two skinks (the azure-tailed and dark-bellied copper-striped skink) may be native or could have arrived unintentionally as a result of human movement. The previous map of these two Emoia species for the Pacific indicated that only the azure-tailed was recorded on Wake, and it missed the early dark-bellied copper-striped skink record (Ineich and Zug, 1991). We detected 1 melanistic azure-tailed skink out of 206 captures using glue boards. This finding indicates the phenotype is present but rare (Bruna and others, 1996b). We cannot know how this information compares to past numbers or if this phenotype is changing frequencies within Wake, limiting our ability to interpret these results. We did not detect any Pacific stump-toed geckos. This species more than likely arrived unintentionally with humans, which is common for this species (Fisher, 1997). The last vouchered specimen was deposited in 1953, and the last verbally reported observation (no photograph or other voucher of any kind) of the Pacific stump-tailed gecko was in 1998. It is possible that this species is still present on Wake in very low numbers; however, it may have been extirpated. The distribution of Pacific stump-tailed geckos in Hawai`i has been presumed to be reduced by the spread of the common house gecko, and it is now rare (McKeown, 1996).
We did not encounter any reptile species not historically recorded at Wake. Although short in duration, the formal nature of our surveys gives us confidence the past record is accurate as to the minimum species present. With regards to the dark-bellied copper-striped skink, which was only recorded in 1923, it is possible that this species is either very rare or secretive and was missed, or it could have been extirpated. There is some likelihood of extirpation given that Pacific rats (Rattus exulans) were reported to be abundant as early as 1568 (Bryan, 1959; Werstein, 1964;), yet the first known reptile voucher was not collected until 1871. The Pacific rat is thought to be mainly vegetarian; however, their diet can expand to include more animal prey, especially when vegetation fails to produce enough food, such as during severe weather events (Mosby and others, 1973). This finding indicates the rats may have indirect and direct effects on reptiles, at least temporarily (as long as they are present). Hoare and others (2007) reported empirical evidence that Pacific rats ecologically displaced Duvaucel’s gecko, reducing capture and recruitment rates in the presence of rats. Towns and others (2007) identified recruitment of tuataras on three islands 4–5 years after eradication of Pacific rats compared to a fourth island that lacked recruitment where rats remained. Towns and others (2007) also saw a large and highly significant increase of body condition (mass) in some cases in the adult tuataras on the three islands where rats had been removed. Fisher and others (2019) reported evidence of recruitment in Pacific iguanas after the eradication of Pacific rats on an island in Fiji. There was no evidence of recruitment in the 14 years prior to their removal. Although tuataras are a distinct lineage and not lizards, their similarities support the documented iguana response to rats. There is likely a negative interaction with rats and resiliency to rebound with rat removal. In New Caledonia, Thibault and others (2017) detected remains of several skink and gecko species in Pacific and black rat guts, further contributing to the base of knowledge regarding rat predation on lizards.
Thought to be introduced in the 1970s, the Asian house rat (Rattus tanezumi) was confirmed to be present at Wake (Siers and others, 2015) but has since been eradicated (Griffiths and others, 2014). Both the Pacific and Asian house rats were confirmed to be eradicated from Peale Islet in 2012. We would expect that any rare reptile species historically present and still persisting at very low levels on Peale would have potentially rebounded and been detected during our surveys.
We did, however, find YCAs, which are an additional likely threat to reptiles at Wake. Yellow crazy ants are widely distributed across Peale and are well known for their effects on many taxa, and reptiles are no exception (O’Dowd and others, 2003; Fisher and Ineich, 2012; Smith and others, 2012; Hoffmann and others, 2014). The history of the YCA and its distribution on Wake is not well known. We did not detect any YCAs on the transect at the north end of Peale, which may be a refuge from YCA; however, this was a short transect set out for about 80 minutes. More searching would be needed to map YCA extent. Although studies elsewhere indicate it is likely, we do not currently (2019) have direct evidence that YCAs are negatively affecting reptile populations at Wake.
Because this was the first systematic survey for reptiles, there was little previous distribution data for this taxonomic group. The 2019 field surveys were an initial assessment of species distributions as well as a general representation of relative abundance. All four species detected were on each islet (appendix D1, figs. D1.4–D1.7) and varied in relative abundance (tables D2, D3). Although we do not know the historical distribution of reptile species on Wake, the current (2019) work indicates that it has not changed considerably from the historical record, with two exceptions: (1) the stump-toed gecko has not been vouchered since 1953, with only one report of a sighting more than two decades ago and (2) the dark-bellied copper-striped skink was recorded in 1923, identified in 1990 by Ivan Ineich, but then not included in the map presented in Ineich and Zug (1991). This species has not been documented again on Wake. The common house geckos and azure-tailed skinks have likely become more widespread and abundant given continued habitat disturbance with storms and new construction during the last several decades. Our surveys indicate that despite extreme and continual modifications to the landscape at Wake and the presence of invasive species known to affect reptiles, the reptile community continues to persist. Time constraints prohibited more exhaustive searching; however, additional adventive and invasive reptile and amphibian species have presumably not recently (in the last several decades) arrived to Wake and become established, with the exception of the blindsnake. Due to time constraints, some HMUs were surveyed less thoroughly than others or not at all. However, the data presented here can serve as a general baseline for future monitoring and management decision making for addressing ecosystem health and in relation to carrying out the installation mission.
Table D1.
Summary and status (including risk and management feasibility based on preliminary risk assessment) of reptile species and dates recorded on Wake Atoll in museum records (with corrected species identifications), reports, and other opportunistic observations, and during U.S. Geological Survey (USGS) reptile surveys in 2019.[—Left][Status: A-I, alien invasive; Nat, native; E*, possibly endemic; A, alien; A-N, alien naturalized. Wake risk: H, high; NA, not applicable/native species; L, low; M, medium. Management feasibility: L, low; NA, not applicable/native species; VL, very low; M, medium. Other abbreviations: —, not applicable; U, no further locality information beyond Wake Island/Atoll; W, Wake Islet; WI, Wilkes Islet; P, Peale Islet. Note that this table should be read left-to-right across both pages]
Table D2.
Sticky glue board transect summary for U.S. Geological Survey reptile surveys at Wake Atoll in 2019.[Trap type: T, tree; L, log; G, ground. Species codes: CRPO, Cryptoblepharus poecilopleurus (Oceania snake-eyed skink); EMCY; Emoia cyanura (azure-tailed skink); EMCY*, melanistic azure-tailed skink; HEFR, Hemidactylus frenatus (common house gecko); LELU, Lepidodactylus lugubris (mourning gecko). Abbreviations: mm/dd/yyyy, month/day/year; No., number; HMU, habitat management unit; YCA, yellow crazy ant; —, not applicable; MDA, Missile Defense Agency]
Table D3.
Summary of U.S. Geological Survey nighttime visual encounter surveys for reptiles on Wake Atoll in 2019.[Species codes: EMCY, Emoia cyanura (azure-tailed skink); HEFR, Hemidactylus frenatus (common house gecko); LELU, Lepidodactylus lugubris (mourning gecko). Abbreviations: mm/dd/yyyy, month/day/year; MDA, Missile Defense Agency]
Table D4.
Summary of reptile specimens collected on Wake Atoll during U.S. Geological Survey surveys in 2019.[Species codes: CRPO, Cryptoblepharus poecilopleurus (Oceania snake-eyed skink); EMCY, Emoia cyanura (azure-tailed skink); HEFR, Hemidactylus frenatus (common house gecko); LELU, Lepidodactylus lugubris (mourning gecko)]
D. References Cited
-
Blom, M.P.K., Matzke, N.J., Bragg, J.G., Arida, E., Austin, C.C., Backlin, A.R., Carretero, M.A., Fisher, R.N., Glaw, F., Hathaway, S.A., Iskandar, D.T., McGuire, J.A., Karin, B.R., Reilly, S.B., Rittmeyer, E.N., Rocha, S., Sanchez, M., Stubbs, A.L., Vences, M., and Moritz, C., 2019, Habitat preference modulates trans-oceanic dispersal in a terrestrial vertebrate: Proceedings of the Royal Society B—Biological Sciences, v. 286, no. 1904, p. 1–10. [Available at https://doi.org/10.1098/rspb.2018.2575.]
-
Bruna, E.M., Fisher, R.N., and Case, T.J., 1996a, New evidence of habitat segregation between two cryptic species of Pacific skinks (Emoia cyanura and E. impar): Copeia, v. 1996, no. 4, p. 998–1005. [Available at https://doi.org/10.2307/1447665.]
-
Bruna, E.M., Fisher, R.N., and Case, T.J., 1996b, Morphological and genetic evolution appear decoupled in Pacific skinks (Squamata: Scincidae: Emoia): Proceedings of the Royal Society B—Biological Sciences, v. 263, no. 1371, p. 681–688. [Available at https://doi.org/10.1098/rspb.1996.0102.]
-
Bryan, E.H., Jr., 1949, Snakes in paradise—One found in Wake: Honolulu Advertiser.
-
Bryan, E.H., Jr., 1959, Notes on the geography and natural history of Wake Island: Atoll Research Bulletin no. 66, p. 1–22. [Available at https://doi.org/10.5479/si.00775630.66.1.]
-
Buden, D.W., and Taboroši, D., 2015, Reptiles of the Federated States of Micronesia: Island Researcher and Education Initiative, 311 p.
-
Buden, D.W., Taboroši, D., Kottermair, M., Jalandoni, A., and Martin, M., 2020, Reptiles of the Northern Marshall Islands: Pacific Science, v. 74, no. 2, p. 189–209. [Available at https://doi.org/10.2984/74.2.8.]
-
Crombie, R.I., and Pregill, G.K., 1999, A checklist of the herpetofauna of the Palau Islands (Republic of Belau), Oceania: Herpetological Monographs, v. 13, p. 29–80. [Available at https://doi.org/10.2307/1467060.]
-
Fisher, R.N., 1997, Dispersal and evolution of the Pacific Basin gekkonid lizards Gehyra oceanica and Gehyra mutilata: Evolution, v. 51, no. 3, p. 906–921. [Available at https://doi.org/10.2307/2411165.]
-
Fisher, R., and Ineich, I., 2012, Cryptic extinction of a common Pacific lizard Emoia impar (Squamata, Scincidae) from the Hawai’ian Islands: Oryx, v. 46, no. 2, p. 187–195. [Available at https://doi.org/10.1017/S0030605310001778.]
-
Fisher, R.N., Niukula, J., Harlow, P., Rasalato, S., Chand, R., Thaman, B., Seniloli, E., Vadada, J., Cranwell, S., Brown, J., Lovich, K., and Thomas-Moko, N., 2019, Community-based conservation and recovery of native species on Monuriki Island, Fiji, in Veitch, C.R., Clout, M.N., Martin, A.R., Russell, J.C., and West, C.J., eds., Island invasives—Scaling up to meet the challenge: Gland, Switzerland, International Union for Conservation of Nature (IUCN), Proceedings of the International Conference on Island Invasives 2017, Occasional Paper SSC no. 62, p. 552–557.
-
Griffiths, R., Wegmann, A., Hanson, C., Keitt, B., Howald, G., Brown, D., Tershy, B., Pitt, W., Moran, M., Rex, K., White, S., Flint, B., and Torr, N., 2014, The Wake Island rodent eradication—Part success, part failure, but wholly instructive, in Vertebrate Pest Conference, 26th, Davis, Calif., 2014, Proceedings: Davis, Calif., University of California, Davis, p. 101–111.
-
Hathaway, S.A., Jacobi, J.D., Peck, R., and Fisher, R.N., 2022, Updates for Wake Atoll biosecurity management, biological control, survey, and management, and integrated pest management plans: U.S. Geological Survey Open-File Report 2022–1067, 56 p., accessed August 26, 2022, at https://doi.org/10.3133/ofr20221067.
-
Hoare, J.M., Pledger, S., Nelson, N.J., and Daugherty, C.H., 2007, Avoiding aliens—Behavioural plasticity in habitat use enables large, nocturnal geckos to survive Pacific rat invasions: Biological Conservation, v. 136, no. 4, p. 510–519. [Available at https://doi.org/10.1016/j.biocon.2006.12.022.]
-
Hoffmann, B.D., Auina, S., and Stanley, M.C., 2014, Targeted research to improve invasive species management—Yellow crazy ant Anoplolepis gracilipes in Samoa: PLoS One, v. 9, no. 4, 10 p. [Available at https://doi.org/10.1371/journal.pone.0095301.]
-
Holway, D.A., Lach, L., Suarez, A.V., Tsutsui, N.D., and Case, T.J., 2002, The causes and consequences of ant invasions: Annual Review of Ecology and Systematics, v. 33, no. 1, p. 181–233. [Available at https://doi.org/10.1146/annurev.ecolsys.33.010802.150444.]
-
Horner, P., 2007, Systematics of the snake-eyed skinks, Cryptoblepharus Wiegmann (Reptilia—Squamata—Scincidae)—An Australian based review: Beagle, Supplement, v. 3, p. 21–202.
-
Ineich, I., 1999, Spatio-temporal analysis of the unisexual-bisexual Lepidodactylus lugubris complex (Reptilia, Gekkonidae), in Ota, H., ed., Tropical island herpetofauna—Origin, current diversity, and conservation: Developments in Animal and Veterinary Sciences, v. 29, Elsevier, p. 199–228.
-
Ineich, I., and Zug, G.R., 1991, Nomenclatural status of Emoia cyanura (Lacertilia, Scincidae) populations in the Central Pacific: Copeia, v. 1991, no. 4, p. 1132–1136. [Available at https://doi.org/10.2307/1446114.]
-
International Union for Conservation of Nature, 2019, Global invasive species database: Invasive Species Specialist Group (ISSG), Species Survival Commission (SSC), International Union for Conservation of Nature (IUCN), online database, accessed November 7, 2019, at http://www.iucngisd.org/gisd/.
-
McDiarmid, R.W., 1994, Preparing amphibians as scientific specimens, in Heyer, W.R., Donnelly, M.A., McDiarmid, R.W., Hayek, L.C., and Foster, M.S., eds., Measuring and monitoring biological diversity—Standard methods for amphibians: Washington, D.C., Smithsonian Institution Press, p. 289–297.
-
McGlynn, T.P., 1999, The worldwide transfer of ants—Geographical distribution and ecological invasions: Journal of Biogeography, v. 26, no. 3, p. 535–548. [Available at https://doi.org/10.1046/j.1365-2699.1999.00310.x.]
-
McKeown, S., 1996, A field guide to reptiles and amphibians in the Hawai’ian Islands: Los Osos, Calif., Diamond Head Publishing Inc., 172 p.
-
Mosby, J.M., Wodzicki, K., and Thompson, H.R., 1973, Food of the kimoa (Rattus exulans) in the Tokelau Islands and other habitats in the Pacific: New Zealand Journal of Science, v. 16, p. 799–810.
-
O’Dowd, D.J., Green, P.T., and Lake, P.S., 2003, Invasional ‘meltdown’ on an oceanic island: Ecology Letters, v. 6, no. 9, p. 812–817. [Available at https://doi.org/10.1046/j.1461-0248.2003.00512.x.]
-
Radtkey, R.R., Donnellan, S.C., Fisher, R.N., Moritz, C., Hanley, K.A., and Case, T.J., 1995, When species collide—The origin and spread of an asexual species of gecko: Proceedings of the Royal Society B—Biological Sciences, v. 259, no. 1355, p. 145–152. [Available at https://doi.org/10.1098/rspb.1995.0022.]
-
Siers, S.R., Shiels, A.B., Goldade, D.A., Volker, S.F., McAuliffe, T.W., Coad, H.L., and Pitt, W.C., 2015, Wake Atoll fish tissue sampling and analysis three years after an island wide rodenticide application: Final Report QA 2241, USDA, APHIS, WS, NWRC. Hilo, HI. 49 p. + appendices.
-
Smith, M.J., Cogger, H., Tiernan, B., Maple, D., Boland, C., Napier, F., Detto, T., and Smith, P., 2012, An oceanic island reptile community under threat—The decline of reptiles on Christmas Island, Indian Ocean: Herpetological Conservation and Biology, v. 7, no. 2, p. 206–218.
-
Thibault, M., Brescia, F., Jourdan, H., and Vidal, E., 2017, Invasive rodents, an overlooked threat for skinks in a tropical island hotspot of biodiversity: New Zealand Journal of Ecology, v. 41, no. 1, p. 74–83. [Available at https://doi.org/10.20417/nzjecol.41.9.]
-
Towns, D.R., Parrish, G.R., Tyrrell, C.L., Ussher, G.T., Cree, A., Newman, D.G., Whitaker, A., and Westbrooke, I., 2007, Responses of tuatara (Sphenodon punctatus) to removal of introduced Pacific rats from islands: Conservation Biology, v. 21, no. 4, p. 1021–1031. [Available at https://doi.org/10.1111/j.1523-1739.2007.00742.x.]
-
Werstein, I., 1964, Wake—The story of a battle: New York, Crowell Company, 145 p.
-
Wetterer J., K., 2005, Worldwide distribution and potential spread of the long-legged ant, Anoplolepis gracilipes (Hymenoptera: Formicidae): Sociobiology, v. 45, p. 77–97.
Appendix D1. U.S. Geological Survey 2019 Reptile Survey Locations and Results
Available at https://doi.org/10.3133/ofr20231066.
Appendix D2. Reptile Specimens Collected by U.S. Geological Survey at Wake Atoll in 2019
Available at https://doi.org/10.3133/ofr20231066.
Appendix D3. Field Guide to the Herpetofauna of Wake Atoll
Available at https://doi.org/10.3133/ofr20231066.
Conversion Factors
International System of Units to U.S. customary units
Temperature in degrees Celsius (°C) may be converted to degrees Fahrenheit (°F) as follows:
°F = (1.8 × °C) + 32.
Director, Western Ecological Research Center
U.S. Geological Survey
3020 State University Drive East
Sacramento, California 95819
https://www.usgs.gov/centers/werc
Publishing support provided by the U.S. Geological Survey
Science Publishing Network, Sacramento Publishing Service Center
Disclaimers
Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Although this information product, for the most part, is in the public domain, it also may contain copyrighted materials as noted in the text. Permission to reproduce copyrighted items must be secured from the copyright owner.
Suggested Citation
Hathaway, S.A., Jacobi, J.D., Peck, R., Backlin, A.R., Hitchcock, C.J., and Fisher, R.N., 2025, Biodiversity surveys of Wake Atoll—Featuring field guides for plants, arthropods, and herpetofauna: U.S. Geological Survey Open-File Report 2023–1066, 302 p., https://doi.org/10.3133/ofr20231066.
ISSN: 2331-1258 (online)
Study Area
| Publication type | Report |
|---|---|
| Publication Subtype | USGS Numbered Series |
| Title | Biodiversity surveys of Wake Atoll—Featuring field guides for plants, arthropods, and herpetofauna |
| Series title | Open-File Report |
| Series number | 2023-1066 |
| DOI | 10.3133/ofr20231066 |
| Publication Date | March 17, 2025 |
| Year Published | 2025 |
| Language | English |
| Publisher | U.S. Geological Survey |
| Publisher location | Reston, VA |
| Contributing office(s) | Western Ecological Research Center |
| Description | x, 302 p. |
| Other Geospatial | Wake Atoll |
| Online Only (Y/N) | Y |



