Effects of Lead Exposure on Birds Breeding in the Southeast Missouri Lead Mining District
Links
- Document: Report (9.8 MB pdf) , HTML , XML
- Data Release: USGS data release - Breeding songbird tissue analyses and metal concentrations in tissues, soil and invertebrates collected near nesting sites within the Southeast Missouri Lead Mining District, 2016–19
- Download citation as: RIS | Dublin Core
Acknowledgments
The U.S. Department of the Interior (DOI) Office of Restoration and Damage Assessment funded this study; the U.S. Department of Agriculture Forest Service (USFS) Northern Research Station provided in-kind support. We thank the private landowners who allowed access to the study sites; numerous personnel from the U.S. Geological Survey (USGS), Missouri Department of Conservation (MDC), Missouri Department of Natural Resources (MoDNR), and U.S. Fish and Wildlife Service (USFWS) for their assistance with site identification, field collection, laboratory analyses, and report preparation; and, specifically, J.E. Hinck (USGS), S.L. Schultz (USGS), M. Wildhaber, (USGS), E. Gramlich (MoDNR), H. Wakefield (MoDNR), K. Knott (MDC), L. Lueckenhoff (USFWS), J. Weber (USFWS), J. Lacoste (USFS), A. Horner Hanley (DOI), and the USGS peer reviewers (R.C. Burner, N.B. Vyas, and R. Tillitt) for their assistance with this report and the associated USGS data release. This report has been reviewed per the USGS Fundamental Science Practices policy.
Abstract
Lead mining in the Southeast Missouri Lead Mining District began in the 1700s and continued for nearly 300 years; the waste piles associated with smelting, mining, and milling of lead ores have released metal residues that have contaminated soil and water in the region. Previous studies in the district have indicated potential harm to wildlife, including birds, because of elevated lead concentrations associated with mining. Exposure to soil-borne lead was correlated with elevated lead concentrations in tissues, inhibition of δ-aminolevulinic acid dehydratase (δALAD), and renal lesions in birds foraging on ground-dwelling invertebrates at contaminated sites (compared to reference sites) in the Southeast Missouri Lead Mining District.
This study assessed reproductive outcomes for songbirds exposed to soil-borne lead in the district, examined the relation between lead concentrations in soils and in tissues of ground-feeding birds and prey species, and compared the results to literature-based toxicity thresholds for lead that are associated with negative effects in birds. Three lead-contaminated sites and three reference sites (with background concentrations of lead and no known mining inputs) were compared in two ways: individually to all other sites or by site type. Additional effects of lead exposure were evaluated by examining concentrations of biomarkers (oxidative stress, lipid peroxidation, and deoxyribonucleic acid damage) in liver tissues, δALAD inhibition, and renal and hepatic microscopic lesions in birds from lead-contaminated and reference sites.
Lead concentrations in soil were site-dependent and were also generally heterogeneous within the lead-contaminated sites. Between 17 and 74 percent of all soil samples at contaminated sites had lead concentrations that exceeded a threshold (1,000 milligrams per kilogram [mg/kg] lead in soil) previously associated with adverse physiological effects in birds in the Southeast Missouri Lead Mining District. Lead concentrations in mixed invertebrates from lead-contaminated sites (282 to 2,230 mg/kg dry weight [dw]) indicated that consuming soil-dwelling prey species is a potential exposure pathway for adult birds and their broods. At lead-contaminated sites, lead concentrations in 40.5 percent of blood samples (adults and their broods) were within a subclinical effects range (0.9 to 2.3 mg/kg dw), and 18.7 percent of samples had lead concentrations that exceeded clinical effects criteria (greater than 2.3 mg/kg dw). In contrast, only 2.6 percent of blood samples from reference sites were within the subclinical effects range for lead; all other blood samples from the reference sites had lead concentrations representative of background concentrations (less than 0.9 mg/kg dw). Subclinical and clinical threshold exceedances for lead concentrations in livers and kidneys were similarly more prevalent at the contaminated sites compared to the reference sites.
Lead concentrations in blood were positively correlated with lead concentrations in soil, livers, and kidneys. Lead concentrations in blood were negatively correlated with δALAD activity; greater than 50 percent of the birds collected at lead-contaminated sites exhibited injury via greater than 50 percent inhibition of δALAD in blood compared to birds at reference sites. Birds with elevated lead concentrations in tissues also exhibited enhanced oxidative stress. Microscopic lesions in the livers and kidneys of birds had similar rates of occurrence at the contaminated and reference sites, and lesion prevalence could not be directly linked to lead exposure. Reproductive success was monitored at 585 nests, and 3 out of 5 species had reduced nest success associated with elevated lead concentrations in soil; habitat measures did not help explain nest success. Reduced nest success may have resulted from greater nest predation resulting from neurological and behavioral effects of lead exposure. Ultimately, these lines of evidence indicate that bird health and reproduction have been negatively affected by exposure to lead-contaminated soils in the Southeast Missouri Lead Mining District.
Introduction
Missouri has a long history of lead mining and processing: the first recorded instance dates back to the early 1700s (Burford, 1978). The lead deposits in the Southeast Missouri Lead Mining District contain the largest known concentration of galena (lead sulfide) in the world (Seeger, 2008), and Missouri was the top global producer of lead in the late 19th through the mid-20th centuries. The Southeast Missouri Lead Mining District has been colloquially divided into the “Old Lead Belt,” and the “New Lead Belt” (alternately named the “Viburnum Trend”) (fig. 1); depletion of lead ore reserves in the Old Lead Belt led to exploration for new deposits in the Viburnum Trend in the 1950s (Seeger, 2008). Both these historical and active mining activities in the Southeast Missouri Lead Mining District have left a legacy of metal contamination in the region; lead from smelting, tailings, and chat has contaminated soils, sediment, biota, and water (Seeger, 2008). Wind, rain, and flooding events continue to release and mobilize lead into various environmental compartments (Besser and others, 2007; Seeger, 2008; Allert and others, 2013; Beyer and others, 2018). The U.S. Fish and Wildlife Service (USFWS), the U.S. Forest Service, and the Missouri Department of Natural Resources, acting in their capacity as trustees for natural resources, started a Natural Resource Damage Assessment and Restoration process at several locations within the Southeast Missouri Lead Mining District (for example, see the Natural Resource Damage Assessment and Restoration case descriptions for the Madison County Mines Superfund Site and the Viburnum Trend Lead Mine and Mill Sites, both located within the Southeast Missouri Lead Mining District, available at https://www.cerc.usgs.gov/orda_docs/CaseDetails?ID=1034 and https://www.cerc.usgs.gov/orda_docs/CaseDetails?ID=274, respectively).
Worldwide, lead exposure and uptake have been documented in greater than 120 species of birds and have caused sublethal and lethal effects (Scheuhammer and Norris, 1995; Haig and others, 2014). Lead has no known physiological function; previous research has demonstrated that ground-foraging birds are at risk of exposure to lead through incidental ingestion of contaminated soil and dust while grooming and feeding, and through other behavioral activities (for example, nest defense and nestling care; Grue and others, 1986; Beyer and others, 1994; Custer and others, 2003; De Francisco and others, 2003; Hansen and others, 2011; Sample and others, 2011; Beyer and others, 2013). Lead causes many of the same effects in birds as it does in other vertebrates after exposure, including mortality, lethargy, vascular damage, digestive tract dysfunction, vision loss, diminished locomotion, and paralysis (Eisler, 1988; Fisher and others, 2006; Pain, 1996; Hoffman and others, 2000).
Lead is primarily excreted through urine and feces; a characteristic external indicator of exposure is watery, green excrement (DeMichele, 1984). However, lead exposure effects can also be nonspecific and difficult to detect (Fallon and others, 2017). For example, lead decreases erythrocyte lifespan and inhibits enzymes required for heme synthesis, including δ-aminolevulinic acid dehydratase (δALAD), which may cause decreased blood hemoglobin concentration and anemia (Pattee and Pain, 2003); hemoglobin concentrations are positively correlated with fitness and reproductive metrics (for example, egg size; Minias, 2015). δALAD inhibition via lead exposure is well characterized, such that field and laboratory toxicity studies commonly use measures of δALAD activity (and its inhibition compared to reference animals) as sensitive biomarkers of lead exposure; moreover, δALAD inhibition is a codified (43 CFR § 11.62(f)(4(v)(D)) measure of injury in Natural Resource Damage Assessment and Restoration cases (Beyer and others, 1988; Mateo and Hoffman, 2001; Gómez-Ramírez and others, 2011; Espín and others, 2015). Injury is considered to have occurred if a species has significantly reduced δALAD activity values at a contaminated site relative to a reference site and a δALAD inhibition of greater than or equal to (≥) 50 percent can be measured. Reduced avian reproduction and increases in numbers of histopathological lesions (in animals at contaminated compared to reference sites) are other codified (43 CFR § 11.62(f)(4(v)(D)) lines of evidence of injury via contaminant exposure.
Further, lead exposure can decrease the lifetime fitness of individual birds because it adversely affects reproductive parameters such as egg hatchability and the development and survival of young (Edens and Garlich, 1983; Buerger and others, 1986; Burger, 1995; Burger and Gochfeld, 2000). Lead uptake has caused neurological damage that adversely affects avian behavior, such as food begging, feeding, depth perception, song learning, territorial aggression, and locomotion (Burger and Gochfeld, 1985, 200030; Ecke and others, 2017; McClelland and others, 2019; Goodchild and others, 2021). Additionally, research suggests that lead uptake is associated with immunosuppression and oxidative damage in birds (Trust and others, 1990; Fair and Myers, 2002; Mateo and others, 2003; Snoeijs and others, 2004, 2005152).
Previous studies have documented lead exposure and uptake in many fish and wildlife species in southeastern Missouri. For example, Niethammer and others (1985) found that specimens of Lithobates catesbeianus (Shaw, 1802) (American bullfrogs), Ondatra zibethicus (Linnaeus, 1766) (muskrats), and Butorides virescens (Linnaeus, 1758) (green herons) collected from contaminated areas in the Southeast Missouri Lead Mining District had greater lead concentrations than specimens (of the same species) collected from reference sites. Allert and others (2009) reported that Orconectes hylas (Faxon, 1890) (woodland crayfish) at contaminated sites had lower survival and lower overall biomass compared to crayfish at reference sites. Fish from waterbodies near active and historical mining sites have also had greater lead concentrations in their blood and muscles than those at reference sites (Schmitt and others, 1993; Gale and others, 2004; Schmitt and McKee, 2016). Beyer and others (2018) documented exposure of Peromyscus leucopus (Rafinesque, 1818) (white-footed mice) to lead in the district; additionally, mice from the contaminated sites had reduced δALAD activities and had signs of oxidative stress.
Lead uptake by wildlife in the Southeast Missouri Lead Mining District likely indicates that at least some fraction of soil-borne lead in the mining-contaminated parts of the district is bioavailable (Stansley and Roscoe, 1996). However, although previous studies indicated uptake and exposure of lead by birds in other lead-contaminated mining districts (that is, the Coeur d’Alene mining district [Blus and others, 1995; Hansen and others, 2011; Johnson and others, 1999]; and the Tri-State Mining District [Beyer and others, 2004]), no investigation had studied the effects of lead exposure on local birds breeding in the Southeast Missouri Lead Mining District until recently (Beyer and others, 2013).
Study Area and Test Species
The purpose of this study was to expand upon that earlier investigation by Beyer and others (2013) by increasing the number of birds examined; and by examining the relations between lead concentrations in soil and in tissues (blood, livers, and kidneys), the associated adverse health effects (for example, δALAD inhibition, oxidative stress, microscopic lesions), and the reproductive outcomes in birds. Beyer and others (2013) determined that ground-foraging birds (northern cardinals and American robins) in the Southeast Missouri Lead Mining District (n=34) had lead concentrations in tissues greater than birds from reference sites (n=39) by factors of 8 in blood, 13 in livers, and 23 in kidneys. In addition, the mean lead concentrations in tissues from the district (Beyer and others, 2013) approached or exceeded adverse effects concentrations reported in the literature (Franson and Pain, 2011), and were greater than concentrations reported in birds from the Coeur d’Alene and the Tri-State Mining Districts (Beyer and others, 2004; Hansen and others, 2011). Birds from the Southeast Missouri Lead Mining District areas experienced a 58- to 82-percent decrease in δALAD activity compared to birds from reference sites, and results for lead in earthworm tissues indicated that birds were exposed to lead via ingestion of soil-dwelling prey species in the district (Beyer and others, 2013).
We examined 8 species of birds (7 songbird species): American robin, eastern bluebird, eastern towhee, Spizella pusilla (A. Wilson, 1810) (field sparrow), Passerina cyanea (Linnaeus, 1766) (indigo bunting), northern cardinal, Molothrus ater (Boddaert, 1783) (brown-headed cowbird) and 1 gamebird species: mourning dove (table 1) at 3 lead-contaminated sites and three references sites in or next to the Southeast Missouri Lead Mining District. These species were selected based on (1) abundance and availability during the study breeding seasons and (2) because they spend considerable time foraging on or near the ground for invertebrates or seeds (Halkin and Linville, 1999; Payne, 2006; Carey and others, 2008; Otis and others, 2008; Greenlaw, 2015) and, as such, have an increased likelihood of ingesting soil-borne lead particles or being otherwise exposed to lead in the environment. We evaluated reproductive metrics for five (eastern bluebird, eastern towhee, field sparrow, indigo bunting, and northern cardinal) of the seven songbird species; the number of nests monitored per year by site and species are shown in table 2.
Table 1.
Life history information for the target avian species in the Southeast Missouri Lead Mining District.[Species included residents (northern cardinal), short-distance migrants (American robin, eastern bluebird, eastern towhee, field sparrow, mourning dove, northern cardinal, brown-headed cowbird), and long-distance migrants (indigo bunting). The territory ranges, habitats, and other life history strategies reproduced here have been reported elsewhere (Howell, 1942; Young, 1956; Halkin and Linville, 1999; Payne, 2006; Carey and others, 2008; Otis and others, 2008; Gowaty and Plissner, 2015; Greenlaw, 2015; Lowther, 2020)]
Table 2.
Number of nests monitored for each species by site and year in the Southeast Missouri Lead Mining District.[Sialia sialis (Linnaeus, 1758) (eastern bluebirds) were monitored in nest boxes installed for this study. Pipilo erythrophthalmus (Linnaeus, 1758) (eastern towhee), Spizella pusilla (A. Wilson, 1810) (field sparrow), Passerina cyanea (Linnaeus, 1766) (indigo bunting), and Cardinalis cardinalis (Linnaeus, 1758) (northern cardinal) build open-cup nests, which were searched for and monitored when found. No nests for Turdus migratorius (Linnaeus, 1766) (American robin) were monitored at any site]
Three lead-contaminated sites (simply called “contaminated sites”) and three reference sites (in other words, sites having background concentrations of lead and no known source of lead-mining inputs) in the Southeast Missouri Lead Mining District were selected for this study (table 3); only two of the three reference sites were evaluated for reproductive measures. The contaminated sites were (1) within the footprint of the Anschutz Mine, (2) in the Big River floodplain, and (3) near Magmont Vent. In this context, “contaminated” sites refer to areas where mining, milling, and smelting processes released lead into the environment, and where lead concentrations in soil exceeded a background concentration of 40 parts per million (ppm; a concentration of 1 ppm, quantified by portable X-ray fluorescence spectrometry [pXRF], is about equivalent to 1 mg/kg analyzed by inductively coupled plasma-mass spectrometry [ICP–MS]). Previous work by Beyer and others (2013) indicated that the Missouri-specific background for lead concentrations in soil is 20 to 62 mg/kg; the selection of 40 ppm as the background concentration represents the approximate midpoint of this background range. Areas that have no history of lead releases from mining, milling, or smelting processes were selected as reference sites.
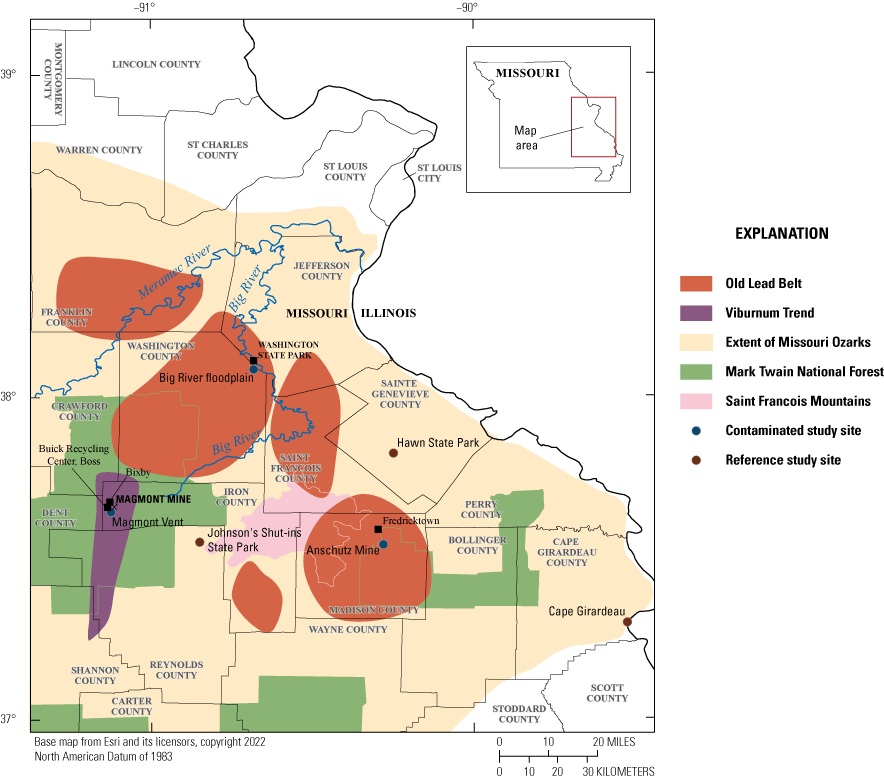
The Southeast Missouri Lead Mining District including subdistricts and avian study sites.
Table 3.
Avian study sites in the Southeast Missouri Lead Mining District.[The global positioning system (GPS) coordinates are provided in the World Geodetic System 84 coordinate system (WGS 84); coordinates are denoted as latitude, longitude, in decimal degrees]
| Site name (fig. 1) | Site type | GPS coordinates | Source of lead contamination |
|---|---|---|---|
| Anschutz Mine | Contaminated | 37.54992, −90.27832 | Lead-cobalt mining and smelting on-site; historical chat piles. |
| Big River floodplain—Washington State Park | Contaminated | 38.08631, −90.67738 | Big River watershed drains the “Old Lead Belt.” |
| Big River floodplain—Private land | Contaminated | 38.10946, −90.68001 | Big River watershed drains the “Old Lead Belt.” |
| Magmont Vent | Contaminated | 37.65603, −91.12093 | Active mining and smelting in the “Viburnum Trend” (or “New Lead Belt”). |
| Johnson’s Shut-ins State Park | Reference | 37.55230, −90.84652 | None known. |
| Hawn State Park | Reference | 37.83194, −90.24746 | None known. |
| Cape Girardeau | Reference | 37.31418, −89.54655 | None known. |
The Anschutz Mine site (table 3) is in Madison County, Missouri, southeast of Fredericktown, Mo., in the Old Lead Belt (fig. 1). The site hosted lead mining and smelting operations between the 1840s and the 1960s (USFWS and Missouri Department of Natural Resources, 2014). The Anschutz Mine site is currently under remediation (2008 to present [2022]) and contained areas of exposed chat piles and areas that were partially capped with uncontaminated soil during this study.
The Magmont Vent site is in the Viburnum Trend (table 3; fig. 1) in Mark Twain National Forest (Iron County, Mo.) and was named for a mine air vent shaft near the inactive Magmont Mine (Bixby, Mo.). The site is near active lead mining and smelting operations. One example of a nearby smelting operation is the Buick Resource Recycling Facility (Boss, Mo.). This facility, which is about 1.6 kilometers (km) southwest of the Magmont Mine, is a former primary lead smelter that has been converted to a secondary smelter; the facility has been operating in that capacity over the last two decades (since the early 2000s). Beyer and others (2013) determined the average lead concentration in soil in this area to be about 1,000 mg/kg dw.
The Big River is a tributary of the Meramec River and flows through multiple counties in the Southeast Missouri Lead Mining District. The river’s drainage area includes much of the Old Lead Belt. Decades of erosion, leaching, and dam failures have resulted in releases of lead-contaminated mine tailings into the river, and historical floods have deposited lead-contaminated sediments along the riverbanks. The Big River floodplain site is in Washington and Jefferson Counties, Mo. (table 3; fig. 1); study areas include a public day-use area in Washington State Park (Desoto, Mo.), and a tract of private land, which is on the Big River about 3.5 km downstream (2.5 km north) from the day-use area. Note that our Big River floodplain study site does not encompass the entirety of the river’s contaminated floodplain areas (see Pavlowsky and others, 2017 for more information about the extent of metal contamination in the watershed). Soil and sediment samples previously collected from the floodplain areas at Washington State Park and nearby river segments had a mean lead concentration of 1,700 mg/kg dw (Beyer and others, 2013; Pavlowsky and others, 2017).
The primary reference sites (table 3; fig. 1) for the reproductive part of this study were Johnson’s Shut-ins State Park (Reynolds County, Mo.) and Hawn State Park (Ste. Genevieve County, Mo.). However, the abundances of target species for lethal sampling at the reference sites were low; therefore, in 2018, Cape Girardeau (Cape Girardeau County, Mo.) was added as a third reference site to increase sample sizes for lethally sampled birds only. As previously mentioned, all reference sites were in areas with no history of lead mining or contamination and were expected to have background concentrations of lead (<40 ppm).
Habitat, geology, soil types, and mixtures of vegetation types were similar among the study sites. All sites were in or near the St. Francois Mountains, which is the topographic and structural high point of the southeast Missouri Ozarks. The study sites are largely underlain by Cambrian or Ordovician dolostone, limestone, sandstone, or siltstone (Horton, 2017). Anschutz Mine, Big River floodplain, Cape Girardeau, and Johnson’s Shut-ins State Park also feature shale; chert is present at Anschutz Mine. Johnson’s Shut-ins State Park includes areas with exposed Precambrian igneous rocks, which locally affect soil type and vegetation; however, bird sampling was not conducted in those areas because we aimed to keep the underlying geology relatively consistent among study sites.
Habitat at Anschutz Mine is a mix of Pinus L. spp. (pine) and Quercus L. spp. (oak) woodlands, old fields dominated by grasses, forbs, and Juniperus virginiana L. (eastern redcedar) tree saplings, and capped and revegetated mine/mill wastes, which are covered by grasses and forbs. The Big River floodplain site consists of mowed fields, old fields, riparian forest, and upland Carya Nutt spp. (oak-hickory) forest on the slopes leading out of the river valley. Magmont Vent is dominated by oak-hickory woodlands and forested uplands with scattered pines; the site is also dissected by mowed power line cuts, a rail line, and a private road. The Hawn State Park site is an upland old field surrounded by oak-hickory and pine forest. Johnson’s Shut-ins State Park consists of old fields and mowed fields surrounded by oak-hickory forest with occasional pines. Photographs of nesting sites and habitat types at the study sites are provided in appendix 1.
Previous Research on Lead Uptake by Wild Birds
Although an extensive body of literature addresses effects of lead in common test and game species (for example, chickens and mallards), few studies address lead exposure, uptake, and toxicity in small, ground-feeding wild birds (for example, songbirds); moreover, no toxicity thresholds link contaminant concentrations (in the environment or in tissues) to effects on songbirds. Incidental ingestion of lead-contaminated soil by ground-foraging birds has been documented near shooting ranges and mining operations (Vyas and others, 2000; Hansen and others, 2011). In the Coeur d’Alene mining district in northern Idaho (not shown), which has a lengthy history of lead mining and smelting, Turdus migratorius (Linnaeus, 1766) (American robin) nestlings (n=10) from mining- and smelting-contaminated areas had lead concentrations in blood ranging from 1.24 to 4.00 milligrams per kilogram (mg/kg) on a dry-weight basis (dw, assuming 78.26 percent moisture; Blus and others, 1995); however, blood samples were not collected from reference birds for comparison. Lead concentrations in liver tissues of hatch year American robins from contaminated areas (n=10) were 0.22 to 17.4 mg/kg dw (assuming 67.74 percent moisture) compared to 0.28 to 0.96 mg/kg dw in juveniles from reference areas (n=2, assuming 67.74 percent moisture; Blus and others, 1995). Young Tachycineta bicolor (Vieillot, 1808) (tree swallows) from contaminated areas (n=11) had lead concentrations in blood ranging from not detected (ND) to 3.45 mg/kg dw and lead concentrations in livers ranging from ND to 1.83 mg/kg dw. Tree swallows from reference areas (n=3) had similar lead concentration ranges in tissues (0.92 to 1.89 mg/kg dw in blood, assuming 78.26 percent moisture; and ND to 9.9 mg/kg dw in livers, assuming 67.74 percent moisture; Blus and others, 1995).
Similarly, Johnson and others (1999) observed that American robins and Melospiza melodia (A. Wilson, 1810) (song sparrows) from contaminated areas in the Coeur d’Alene mining district exhibited δALAD inhibition of 51 to 75 percent compared to birds from reference areas. In other words, δALAD activity in song sparrows was reduced from 305 units of δALAD activity at the reference area to 149 activity units at the contaminated area (51 percent); δALAD activity in American robins was reduced from 302 units at the reference site to 76 units at the contaminated site (75 percent). Additionally, the mean lead concentration in livers of song sparrows from contaminated areas (5.98 mg/kg dw, assuming 67.74 percent moisture; n=5) was about twentyfold greater than that in song sparrows from reference areas (0.30 mg/kg dw, assuming 67.74 percent moisture; n=4).
A subsequent study of ground-feeding songbirds (American robins, song sparrows, and Catharus ustulatus [Nuttall, 1840] [Swainson’s thrushes]) in the Coeur d’Alene mining district (Hansen and others, 2011) found that birds from reference sites had lead concentrations in blood less than (<) 0.19 mg/kg dw; comparatively, birds from moderately and highly contaminated sites had elevated lead concentrations in blood (1.09 to 2.06 mg/kg dw, respectively). Mean lead concentrations in bird livers were 0.27 mg/kg dw at the reference sites and 6.56 to 15.8 mg/kg dw at the contaminated sites. Birds at the contaminated sites experienced δALAD inhibition of 31 to 71 percent compared to birds at the reference site. Regression analyses of the soil ingestion rate and the lead concentrations in ingesta and soil indicated that lead concentrations in soil at a study site approximates the lead concentrations ingested by birds (Hansen and others, 2011). As a result, Sample and others (2011) concluded that the lead concentrations in blood and livers of birds in the Coeur d’Alene mining district were directly correlated to the lead concentrations in soil.
In the Tri-State Mining District in southwestern Missouri, northeastern Oklahoma, and southeast Kansas (not shown), Beyer and others (2004) sampled blood, livers, and kidneys of five bird species (American robin; Cardinalis cardinalis [Linnaeus, 1758] [northern cardinal]; Riparia riparia [Linnaeus, 1758] [bank swallow]; Stelgidopteryx ruficollis [Vieillot, 1817] [southern rough-winged swallow]; Toxostoma rufum [Linnaeus, 1758] [brown thrasher]) for chemical, biochemical, and histopathological analyses. Birds from the Tri-State Mining District had greater mean lead concentrations in livers (4.2 to 9.3 mg/kg dw), kidneys (6.2 to 20 mg/kg dw), and blood (1.1 to 1.7 mg/kg dw) compared to birds from the reference sites, which were in Maryland (liver, <0.02 to 5.1 mg/kg dw; kidney, 0.081 to 8.9 mg/kg dw; blood, <0.04 to 0.86 mg/kg dw; Beyer and others, 2004). Mean lead concentrations in tissues of American robins from the Tri-State Mining District were as much as 2.2-fold greater than in robins from the reference sites; lead concentrations in northern cardinals at the Tri-State Mining District were as much as 23-fold greater than in cardinals from reference sites. Tree swallows at the Tri-State Mining District had lead concentrations in tissues 27 to 210 times greater than concentrations in swallows from reference sites. Additionally, all northern cardinals (n=6) and American robins (n=6) sampled in the Tri-State Mining District had greater than (>) 50 percent δALAD inhibition compared to birds from reference sites (n=10: 6 cardinals and 4 robins); 4 individuals (2 robins, 1 cardinal, and 1 thrasher) from the Tri-State Mining District had >90 percent δALAD inhibition. Although microscopic lesions in tissues could not be definitively linked to lead toxicity, several individuals (n=6 of 34) from the Tri-State Mining District had lead concentrations in tissue that exceeded literature-based toxicity thresholds (that is, external signs of lead poisoning occur when lead concentration in blood is 2.6 to 5.2 mg/kg dw or hepatic lead is 20 to 50 mg/kg dw; Pain, 1996).
Effects of Lead Exposure on Avian Recruitment
Adult birds that have accumulated lead in their tissues (for example, in bone, blood, and kidneys) can excrete lead from their body via multiple physiological mechanisms. For females, egg laying is a potential route of excretion that can reduce the lead burden of the female but may subsequently expose the offspring to lead. Maternal transfer of lead to eggs has been documented in many avian species, including Anas platyrhynchos (Linnaeus, 1758) (mallards) (Vallverdú-Coll and others, 2016), Branta canadensis (Linnaeus, 1758) (Canada geese) (Tsipoura and others, 2011), Streptopelia roseogrisea (Sundevall, 1857) (ringed turtle doves) (Kendall and Scanlon, 1981), Larus argentatus (Pontoppidan, 1763) (herring gulls) (Burger and Gochfeld, 1993), and four species of terns (Maedgen and others, 1982; Burger and Gochfeld, 1991, 199327). For example, 1 study of Ficedula hypoleuca (Pallas, 1764) (pied flycatchers) nesting near lead smelter operations determined that the median lead concentration in egg contents was 10 times greater at contaminated sites than at reference sites (Nyholm, 1998). Lead concentrations in feathers of adult female Sterna hirundo (Linnaeus, 1758) (common terns) have correlated to concentrations in their eggs (Burger and Gochfeld, 1991), which further indicates maternal transfer of lead to offspring.
Maternal transfer of lead to eggs can reduce egg hatchability. For example, Buerger and others, 1986) observed an about 16-percent reduction in hatchability of eggs laid by lead-exposed (one lead pellet; 72 milligrams [mg] of lead) female Zenaida macroura (Linnaeus, 1758) (mourning doves) compared to unexposed control females; the authors hypothesized that maternal transfer of lead caused increased rates of early embryonic mortality. Additionally, embryo exposure to metals in the egg may inhibit early nestling brain development and motor skills (Grue and others, 1986; Nyholm, 1998; Müller and others, 2008); however, the exact effects of maternally mediated lead concentrations seem to be species dependent. For example, Nyholm (1998) found that maternal exposure to lead did not significantly affect breeding results (that is, number of fledged nestlings per egg) of pied flycatchers, nor was the health of the offspring affected by maternal-mediated lead concentrations.
After hatching, young birds are exposed to lead principally through direct ingestion of lead-contaminated food items and soil. Lead concentrations in the blood of nestlings and adult birds have reflected the lead concentrations in soil of their environment (Nyholm, 1998); lead concentrations in blood increase with increases of lead concentrations in soil (Roux and Marra, 2007). Altricial nestlings, which hatch blind and have little or no down, are unable to leave the nest for 2 to 3 weeks after hatching and rely on prey species provided by parents (Burger and Gochfeld, 1993; Nyholm, 1998; Janssens and others, 2003). Because of their exposure at early ages, altricial young also can have greater sensitivity to lead than adults of the same species (Hoffman and others, 1985b; Grue and others, 1986; Scheuhammer, 1987); nestlings are particularly useful as sentinels of adverse local conditions because their uptake and exposure closely reflects local contaminant bioavailability because of the limited foraging ranges of the parents during the breeding season (Burger and Gochfeld, 1993; Nyholm, 1998; Janssens and others, 2003).
The effects of lead exposure on nestlings are similar to effects on adults and include decreased hemoglobin, inhibited δALAD activity (Grue and others, 1984, 198672; Hoffman and others, 1985b), and decreased cellular immune response (Vallverdú-Coll and others, 2016). For example, Grue and others (1986) determined that adults and nestlings of Sturnus vulgaris (Linnaeus, 1758) (European starlings) exposed to lead in soil (56–410 mg/kg dw) and invertebrates (140–1,200 mg/kg dw) had decreased δALAD activity, but nestlings also experienced decreased brain weights, and decreased hematocrit and hemoglobin concentrations, compared to control birds (soil: 16–34 mg/kg dw lead; invertebrates: 3.2–58 mg/kg dw). Similarly, Falco sparverius (Linnaeus, 1758) (American kestrel) nestlings that were fed a diet containing 125 mg/kg dw lead (as metallic lead or lead acetate) had decreased δALAD activity, hemoglobin concentration, and growth rate, and had increased mortality and delayed development, whereas adult kestrels fed a comparable control diet experienced no change in hematocrit, body weight, or survival (Custer and others, 1984; Hoffman and others, 1985a, 1985b82).
Additionally, altricial young are generally less tolerant of lead than their precocial counterparts (Hoffman and others, 1985b; Grue and others, 1986; Scheuhammer 1987). For example, the altricial kestrel nestlings described in the previous paragraph had multiple exposure effects, including reduced growth, when fed diets containing 125 mg/kg dw lead (Hoffman and others, 1985a), whereas growth impairment in precocial species, such as young chickens and Coturnix japonica (Temminck and Schlegel, 1848) (Japanese quail), was not apparent until the lead concentration in their diets was increased to 500 or 1,000 mg/kg (Stone and others, 1977; Franson and Custer, 1982). Differential growth and feeding rates may affect nestling tolerance to lead. Growth rates of altricial chicks are three to four times greater than those of precocial chicks, so altricial young may be fed lead-contaminated prey at a faster rate than precocial young feed themselves (Ricklefs, 1984). Potential differences in sensitivity to lead between altricial and precocial bird species are particularly of note for this study because the birds in this study are altricial, whereas literature-based toxicity thresholds were derived from studies conducted using precocial species such as chickens and ducks. In this way, the birds in this study may be substantially more sensitive to lead contamination than indicated by the established avian toxicity benchmarks (Franson and Pain, 2011).
Lead exposure has also been correlated with decreased body weight, increased nestling mortality (Hoffman and others, 1985a, 1985b82; Burger and Gochfeld, 1996; Vallverdú-Coll and others, 2016), and decreased nestling weight at fledging, which is a vital part of juvenile survival and fitness (Magrath, 1991; Gebhardt-Henrich and Richner, 1998; Naef-Daenzer and others, 2001). These effects may be caused by the harmful effects of lead exposure on brain development and function during early avian development. The brains of young birds are particularly sensitive to the effects of lead exposure; for example, decreased brain weight in lead-exposed young birds has been documented in at least three species (American kestrels, European starlings, and pied flycatchers) in studies performed in the laboratory and in the wild (Hoffman and others, 1985a; Grue and others, 1986; Nyholm, 1998). Developing male Taeniopygia guttata (Vieillot, 1817) (zebra finches) exposed to lead in drinking water (1,000 micrograms per liter) had impaired song learning, reduced song nuclei, and altered sexual traits, causing reduced attention from females (Goodchild and others, 2021). In addition to physiological effects, lead (100 mg/kg as lead acetate) can adversely affect neurobehavioral and cognitive development and learning in nestlings (Burger, 1998; Burger and Gochfeld, 2000, 200531). Burger and Gochfeld (1996) studied these effects in the field by injecting lead acetate (100 mg/kg in sterile water) into 1 Larus argentatus (Pontoppidan, 1763) (herring gull) nestling in each of 22 broods. They determined that the lead-injected nestlings were smaller, less vigorous, and less able to compete for food with their unexposed siblings; subsequently, they weighed less by the age of 16 days, and increased parental care was required to overcome growth and behavioral deficits in the lead-exposed chicks. Locomotion, thermoregulation, begging, feeding, and response behaviors were also negatively affected in the lead-injected nestlings compared to the control nestlings. Similarly, lead-dosed (0.15 gram [g] lead) wild Sialia mexicana (Swainson, 1832) (western bluebird) nestlings had a decrease in the behavioral response of righting themselves after being placed on their backs relative to within-brood controls (Fair and Myers, 2002).
Lead exposure in nestlings can also delay the date of fledging and reduce overall rates of fledging success. For example, lead-injected herring gull nestlings had significantly lower fledging success than control nestlings (Burger and Gochfeld, 1996). In a study of Parus major (Linnaeus, 1758) great tits nesting along a contamination gradient (emanating from a smelter), fledging occurred substantially later at sites polluted with lead and other heavy metals (Janssens and others, 2003) compared to nestlings at study sites farther away from the smelter. Many studies have documented that fledging date affects juvenile survival and recruitment; individuals that leave the nest earlier generally have higher survival rates (Visser and Verboven, 1999; Monrós and others, 2002). Ultimately, adverse effects on recruitment may lead to population declines and losses of avian ecosystems services (including pest control, seed dispersal, and pollination; Gaston, 2022).
Study Scope
This study aimed to develop multiple lines of evidence of sublethal lead exposure, uptake, effects, and injury related to soil-borne lead for birds nesting at metals-contaminated sites compared to their counterparts at reference sites in the Southeast Missouri Lead Mining District. The specific objectives were the following: (1) determine lead exposure and uptake in birds breeding within the district by quantifying lead concentrations in blood, liver, and kidney tissues, and by evaluating lead concentrations in local soils and terrestrial invertebrates; (2) compare lead concentrations in bird tissues from contaminated sites to bird tissues from reference sites and to literature-based thresholds for avian toxicity; (3) assess sublethal physiological harm using δALAD activity, biochemical indicators of oxidative stress and deoxyribonucleic acid (DNA) damage, and microscopic examinations of kidney and liver tissues for evidence of lesions associated with lead exposure; and (4) determine if reproductive endpoints (clutch size, hatching success, number of young fledged, and nest survival) of five focal bird species were correlated to lead concentrations in blood and in local soils. All data (metadata and digital datasets) from this study are available in a U.S. Geological Survey (USGS) data release (Cleveland and others, 2023), per USGS data management policy, at https://doi.org/10.5066/P9RV1D60.
Methods
For this study, nests (open-cup and nest box) were monitored, and lethal and nonlethal sampling methods were used to assess whether exposure to lead-contaminated soils adversely affected physiological functions (for example, antioxidant defense and heme synthesis) and reproduction of birds (that is, clutch size, number of young hatched, nestling weight, number of young fledged, nest success, and daily nest survival rates) during three breeding seasons (2016, 2017, and 2018). We performed field reconnaissance before starting the study to determine the general suitability of habitat, characterize the diversity and abundance of bird species, and identify suitable locations for the placement of eastern/western bluebird nest boxes at each study site; note that the nest box plans (North American Bluebird Society, 2016) are for eastern and western bluebirds, but only eastern bluebirds were part of this study.
Bird Tissue Sampling
We collected blood (lethal or nonlethal), liver, and kidney samples from adult birds; blood samples (nonlethal) were also collected from nestlings and composited at the brood level for analyses (table 4). We quantified lead concentrations (blood, livers, and kidneys), δALAD activity (blood), and biochemical indicators of oxidative stress and DNA damage (livers) to assess lead exposure, uptake, and injury of birds. The livers and kidneys were examined for microscopic lesions typically associated with lead toxicoses. Lead concentrations in blood are one of the most-used measurement endpoints for assessing recent exposure to lead (days to weeks; Burger and Gochfeld, 1997; Beyer and others, 2004; Johnson and others, 2007; Buekers and others, 2009; Hansen and others, 2011) because lead concentrations in blood generally represent recent exposure via ingestion and gastrointestinal absorption (Vyas and others, 2001; Franson and Pain, 2011), whereas elevated lead concentrations in other tissues, including kidneys, livers, feathers, and bone, indicate long-term or chronic exposure (months to years). We targeted females because they produce the eggs and generally provide more parental care than the males; however, we sampled males when we were unable to capture the female of the breeding pair. Adult eastern bluebirds were captured using nest box traps (Friedman and others, 2008) or mist nets, whereas the adults of all other species (eastern towhee, field sparrow, indigo bunting, northern cardinal) were captured in their breeding territories using song playback and mist nets or funnel traps.
During lethal sampling (American robin, northern cardinal, mourning dove, brown-headed cowbird), we first collected blood samples for analyses of lead concentration, microhematocrit, and δALAD activity, and then euthanized the animals for collection of liver and kidney tissues (table 4). Individuals were euthanized in the field by inhalant carbon dioxide from a gas cylinder (Fair and others, 2010). Blood for lead analysis was collected from the cutaneous ulnar vein (punctured using a 26-gauge, 0.5-inch-long needle) using nonheparinized capillary tubes; the blood was then immediately transferred into pre-labeled, pre-cleaned glass screw-top test tubes. The test tubes were left capped until the sample was drawn and were re-capped immediately after filling to reduce or eliminate background contamination (app. 2). Samples were stored on ice in a cooler while in the field, and then frozen (−20 °C) until further processing. For δALAD analysis, blood was collected into heparinized capillary tubes and then immediately transferred into cryovials, frozen in liquid nitrogen in the field, and stored at −80 °C until further processing and analysis. Blood samples for microhematocrit were collected in heparinized capillary tubes; tubes were immediately placed on ice in a cooler while in the field and processed by centrifugation within 5 hours of collection.
The liver and kidneys were immediately dissected using clean, stainless steel dissecting tools. Tissues were divided into multiple sections, one each for histopathology (kidney and liver), lead analysis (liver and kidney), and oxidative stress and DNA damage (liver). The sections for each analysis were taken from consistent locations within each organ to standardize sampling protocols among individuals to the extent possible. A liver sample for histopathology consisted of an about 5- by 5-millimeter piece of the liver that was placed into a pre-labeled, plastic sample container containing 1:10 neutral buffered formalin (NBF). One lobe of the kidney was also added to this same sample cup. Histopathology samples were kept submerged in NBF at room temperature until shipping. Immediately before shipment to the pathology laboratory, the NBF was drained, and each tissue was wrapped in a NBF-soaked paper towel and sealed back into the labeled container. Tissues for lead analyses consisted of one lobe of kidney and an about 0.25-g ww section of liver, which were placed into two separate, pre-labeled and acid-washed plastic vials and stored on ice in the field. Samples were kept frozen (−20 °C) until further processing. Finally, an about 125-milligram (mg) ww section of liver was placed into a cryovial and immediately frozen in liquid nitrogen for analyses of oxidative stress and DNA damage biomarkers. Samples were kept frozen at −80 °C until further processing. All dissecting tools and surfaces were thoroughly cleaned with isopropyl alcohol between dissections.
Adults captured for nonlethal blood sampling were fitted with a USGS aluminum band, and we recorded standard morphometric measurements (weight, wing chord, tarsus length, bill length, and fat score). As before, we then collected blood from the cutaneous ulnar vein using nonheparinized capillary tubes; blood from the capillary tubes was immediately transferred into pre-labeled, pre-cleaned glass screw-top test tubes. Samples were stored on ice in a cooler while in the field and subsequently frozen (−20 degrees Celsius [°C]) until further processing. The volume of blood collected from individuals was no more than 1 percent of the body weight of the individual, in adherence to State and Federal guidelines and the recommendations of the Ornithological Council (Fair and others, 2010). Adult birds were released near the original site of capture after completing blood sampling.
Nestlings (table 4) were hand captured late in the nestling period, about 1 to 3 days before the expected day of fledging; the date of fledging was based on frequent observation of the nests (three to four times per week; Ralph and others, 1993). Each nestling was fitted with a USGS aluminum band and measured for tarsus length and body weight. The protocol for nestling (nonlethal) blood collection and sampling was the same as for adults; thus, a smaller volume of blood was collected from each individual nestling based on body weight. Therefore, blood samples from all nestlings within a brood were composited into a single, composite sample that represented the whole brood. We only included blood from conspecific nestlings, and not from cowbirds, in brood samples from nests that had been parasitized by cowbirds.
All bird capture and tissue collection occurred in adherence with Federal bird banding regulations (50 CFR parts 10, 13, and 21; R. Brasso 23903, sub-permittees M. Roach and K. Hixson); annual Missouri State collection permit regulations for 2016, 2017, and 2018 (R. Brasso: #16685, 17314, 17753 and M. Roach: #16778, 17268, 17657, respectively); and USFWS Scientific Collecting Permit regulations (D. Mosby, MB210154–0). Protocols were reviewed and approved by the Institutional Animal Care and Use Committees of Southeast Missouri State University and the U.S. Geological Survey’s (USGS) Eastern Ecological Science Center (formerly the Patuxent Wildlife Research Center).
Soil and Terrestrial Invertebrate Sampling
We also collected and analyzed soil and prey species (earthworms and beetle larvae) from the areas directly surrounding nest locations for lead concentrations to document a potential exposure pathway for ground-feeding birds. We hypothesized that exposure to lead-contaminated soils would cause adverse effects at the molecular, tissue, organ, and whole animal level in a dose-dependent manner, and thus potentially affect reproduction. Soil samples were either analyzed onsite or collected for offsite analyses of lead concentration at each site either immediately before (January to early March of 2016, 2017, 2018, or 2019) or after (August to November of 2016, 2017, or 2018) the breeding season. These efforts were offset from the breeding season to avoid disrupting nest building, egg laying, hatching, and other reproductive behaviors. The soil samples were either analyzed for lead onsite using pXRF or collected in plastic zipper bags and returned to the laboratory for offsite lead analyses by pXRF. All pXRF analyses followed the manufacturers’ instructions (similar to U.S. Environmental Protection Agency [EPA] method 6200; EPA, 2007). Quality-control (QC) measures (app. 2) for the pXRF units involved analyses of known standards and silicon dioxide blanks and the use of duplicate samples.
At the reference sites (table 3), we performed an initial round of quantification of lead in soil; those preliminary results indicated that neither reference site warranted intensive soil sampling because the lead concentrations in the reference soils were relatively low and homogeneous across the sites. Therefore, at the reference sites, composite soil samples were collected on a grid independent of exact location of bird nests. All reference soils were analyzed offsite. In contrast, lead concentrations in soil at the contaminated sites (table 3) were expected to be significantly more heterogeneous than the reference sites. Therefore, the lead concentrations of soils at the contaminated sites were characterized on a spatial basis. In this way, most soil sampling locations at the contaminated sites were within 100 m of each nest or nest box at 20-m intervals along two perpendicular transects intersecting nests. If nests were within 50 m of each other, one long transect was designed to intersect multiple nests. Discrete duplicate soil samples were collected from a subset of the onsite sampling points, which were analyzed offsite for lead concentrations to confirm the onsite results; these QC samples were collected using clean plastic or stainless-steel scoops at every third onsite sampling point along the transect (app. 2). Samples were similarly collected for offsite analyses when onsite conditions were too wet; additional discussion of QC measures for analyses of lead in soils, including the use of duplicate sampling and the collection of soils for offsite analyses (because of saturation with water), is in appendix 2. Site-specific information, including global positioning system coordinates, and date and time of sampling, was recorded at each sampling point. Between pXRF readings, the plastic shield that separates the pXRF window from the soil was cleaned with a low-lint cellulose fiber wiper, damp cloth, or disposable paper towel.
Clitellata spp. (earthworms) and beetle larvae (that is, Coleoptera spp. [Linnaeus, 1758] [grubs]) representing potential invertebrate prey species of the target bird species were collected for lead analysis in 2016. A total of 23 composite samples (consisting of about 90 percent earthworms and 10 percent beetle larvae by wet weight [ww]) were collected; sample sizes were n=5 at Hawn State Park, n=3 at Johnson’s Shut-ins State Park, n=3 at Anschutz Mine, n=9 at Big River floodplain, and n=3 at Magmont Vent. The weights of the invertebrate composite samples ranged from about 1 to 20 g ww, depending on invertebrate abundance at each site. Samples were collected from random locations (but locations at which lead concentrations in soil were analyzed using onsite pXRF) throughout the study sites to represent a range of lead concentrations in prey species. Invertebrates were collected from the top 15 cm of soil (in other words, the depth to which they would be most accessible to foraging birds) by digging with clean trowels; any invertebrates found were hand-transferred to plastic zipper bags, composited by site, and frozen until processing and analysis. The invertebrate samples were not depurated or rinsed before analyses to approximate incidental soil ingestion and dietary exposure to birds. We noted that, in general, the Big River floodplain site and areas near human use (that is, State park campgrounds) had considerably more worms than remote, upland sites such as Magmont Vent, Anschutz Mine, and areas within the reference sites that are remote and have less human use.
Table 4.
Summary of total numbers of samples by sample type, study site, and species in the Southeast Missouri Lead Mining District.[δALAD, δ-aminolevulinic acid dehydratase]
Numbers of adult blood samples for lead analyses include individuals sampled by either lethal or nonlethal methods. Brood blood samples for lead analyses were nonlethal composites. Blood samples for δALAD activity analyses were collected via lethal sampling only. Liver and kidney samples (lethal sampling) were collected for analyses of lead, and oxidative stress and deoxyribonucleic acid damage markers, as well as for histopathological examination. If a sample type was not collected at any study site for a species, it was omitted from this table for brevity.
Two American robins were age class hatching year (HY); the livers and kidneys of these individuals were included in the analyses, but the blood samples were excluded so that only adults classed as after hatching year (AHY) were analyzed.
A total of nine blood samples were collected from American robins for δALAD analysis at Cape Girardeau, but one sample did not have hematocrit measured in the field. Therefore, δALAD activity could not be calculated for that sample; thus, the final sample count for that site and species was eight.
A total of seven blood samples were collected from brown-headed cowbirds at Magmont Vent for δALAD analysis. One sample was clotted but could still be pipetted and was therefore assayed. Although the sample was analyzed for δALAD, the result was excluded from the statistical analyses.
Analyses of Lead in Ground-Dwelling Invertebrates
Invertebrate samples were freeze-dried, homogenized, microwave-digested in high purity nitric acid and hydrogen peroxide (similar to EPA method 3050B; EPA, 1996), and analyzed for lead on a dw basis using ICP–MS (PerkinElmer Elan DRC–e or PerkinElmer NexION 2000, Waltham, Massachusetts; similar to EPA method 6020B; EPA, 2014) at the USGS Columbia Environmental Research Center (Columbia, Mo.). The QC measures for ICP–MS analyses included the use of a minimum of four calibration standards (traceable to the National Institute of Standards and Technology, Gaithersburg, Maryland) and a calibration blank, second-source continuing calibration standards and blanks, laboratory control solutions, certified reference materials, method and analytical duplicates and spikes, interference check solutions, and analytical dilutions. The QC results are summarized in appendix 2.
Analyses of Lead in Bird Tissues
Blood, liver, and kidney samples (table 4) were lyophilized before analysis; percent moisture in each sample was determined gravimetrically by weight loss on freeze-drying at the USGS Columbia Environmental Research Center. Blood samples were acid-digested in high-purity nitric acid and hydrogen peroxide in the collection tubes on a hot block (similar to the method used by Brumbaugh and others, 2005). Freeze-dried liver and kidney tissues were acid-digested with microwave-assisted heating in high-purity nitric acid and hydrogen peroxide (similar to EPA method 3050B; EPA, 1996). Sample digests were analyzed for lead concentrations using ICP–MS (similar to EPA method 6020B; EPA, 2014). All lead tissue results were reported in milligrams per kilogram on a dw basis. QC measures for ICP–MS analyses of lead in the blood, liver, and kidney samples included the use of at least four National Institute of Standards and Technology-traceable calibration standards plus a calibration blank, second-source continuing calibration standards and blanks, laboratory control solutions, certified reference materials, method and analytical duplicates and spikes, interference check solutions, and analytical dilutions (app. 2).
Determination of δALAD Activity
δALAD activity in blood samples (n=52, table 4) was determined by the method of Burch and Siegel (1971) as modified by Pain (1987) at the USGS Eastern Ecological Science Center (Laurel, Md.). The sample volume requirements for the δALAD assay were scaled down to use a volume of 25 microliters (µL) whole blood for analysis, and all samples were analyzed in duplicate. Briefly, samples were incubated pre-assay for 10 minutes at 38 °C. Freshly prepared 0.01 molar (M) aminolevulinic acid substrate (pH 6.65) was then added to each sample tube, and the mixtures were incubated for an additional 60 minutes. The assay was terminated by the addition of 10-percent trichloroacetic acid. The incubation tubes were then centrifuged at 1,500× g, and the supernatant was reacted with Erlich’s reagent. The absorbance of the reacted supernatant was measured at 555 nanometers, background corrected using the absorbance at 630 nanometers, and pathlength corrected at 90-second intervals for as much as a total of 21 minutes using a FLUOstar Omega microplate reader (BMG LABTECH Inc., Cary, North Carolina). Optimal absorbance occurred at about 15 minutes after adding Erlich’s reagent. Units of δALAD activity correspond to ΔA/RBC/h, where A is the background-corrected absorbance at 555 nanometers; RBC is the hematocrit, in milliliters; and h is the time, in hours (Burch and Siegel, 1971). Hematocrit was measured in the packed red cell fraction on a micro-hematocrit capillary tube reader after centrifugation (IEC International Micro-Capillary Centrifuge, Boston, Mass.) of a plugged capillary tube at 13,460× g for 3 minutes. The packed red cell fraction was measured on a micro-hematocrit capillary tube reader. Preliminary assays to verify the δALAD method, and details of the QC measures, are described in appendix 2. Samples were assayed in 3 batches (consisting of 6 samples, 18 samples, and 28 samples, respectively); chicken blood was assayed multiple times in each batch as a QC sample. No samples had activity or absorbance below the limit of detection (LOD; app. 2, table 2.1).
Analyses of Oxidative Stress Indicators and DNA Damage
Liver samples (n=53) were analyzed at the USGS Eastern Ecological Science Center for biomarkers of oxidative stress, namely total sulfhydryl (TSH), total glutathione (tGSH), reduced glutathione (GSH), oxidized glutathione (GSSG), protein bound sulfhydryl (PBSH), lipid peroxidation (thiobarbituric acid reactive substances [TBARS]), and DNA damage (8-hydroxy-2′-deoxyguanosine [8-OH-dG]). We also measured the ratio of GSSG to GSH (GSSG:GSH). For TSH, tGSH, GSH, GSSG, PBSH and TBARS analyses, an about 100-mg ww piece of frozen liver sample was placed in a 1.5-milliliter tube on wet ice and mixed with a 0.6-milliliter volume of 1× phosphate-buffered saline (PBS, pH 7.4; Fisher BioReagents, Waltham, Mass.). Stainless steel beads (0.9- to 2.0-millimeter blend; Next Advance, Inc., Troy, New York) were added to each tube at 1.3 times the weight of the liver piece; the samples were homogenized using a Bullet Blender (Next Advance, Inc., Troy, N.Y.). The homogenate was centrifuged at 10,000× g for 10 minutes at 4 °C and the supernatant saved. Two aliquots of each supernatant were diluted in 1× PBS at concentrations of 100 and 25 milligrams per milliliter (mg/mL), and separately frozen at −80 °C until analysis. Concentrations of GSH and tGSH were measured using the DetectX Glutathione Fluorescent Detection Kit (Arbor Assays, Ann Arbor, Michigan) following the manufacturer’s protocol. The 25 mg/mL supernatant was thawed on ice and diluted to 12.5 mg/mL in 1× PBS for the analysis of brown-headed cowbird, northern cardinal, and mourning dove samples; the 25 mg/mL supernatant was similarly thawed on ice but diluted to 6.25 mg/mL in 1× PBS for American robin samples.
Concentrations of TSH were analyzed using the Measure-iT Thiol Assay Kit (Invitrogen Molecular Probes, Eugene, Oregon) following the manufacturer’s instructions. For this assay, the 25 mg/mL supernatant from brown-headed cowbird, northern cardinal, and mourning dove samples was diluted to 8.33 mg/mL in 1× PBS; the 25 mg/mL American robin supernatant was diluted to 5.0 mg/mL in 1× PBS. TBARS concentrations were determined using the QuantiChrom TBARS Assay Kit (Bioassay Systems, Hayward, California) following the manufacturer’s instructions. For TBARS, the 100 mg/mL supernatant was used for all four species, although for some American robin samples, the supernatant had to be diluted to 12.5 mg/mL in 1× PBS. Concentrations of PBSH were calculated as the difference between TSH and GSH concentrations. GSSG (that is, [tGSH−GSH]/2), and the ratio of GSSG:GSH were calculated using the measured endpoints. Total protein concentrations were determined with a 5 micrograms per microliter supernatant (in 1× PBS) using the Pierce BCA Protein Assay Kit (ThermoFisher Scientific, Waltham, Mass.). Each sample was assayed in duplicate; any sample for which the coefficient of variation of duplicates was >10 percent was re-analyzed. No duplicate coefficient of variation was >10 percent after re-analysis. Two reference samples were run on each plate to monitor interassay variability; additional QC details are provided in appendix 2.
DNA was extracted from about 5 mg of liver using the Gentra Puregene Tissue Kit (Qiagen, Gaithersburg, Md.). The concentration and purity of DNA were determined using a NanoDrop 8000 microvolume ultraviolet-visible spectrophotometer (Thermo Fisher Scientific, Wilmington, Delaware). The samples were normalized to 15 micrograms of DNA per 100 µL in hydration solution (10 millimolar [mM] tris[hydroxymethyl]aminomethane, 1 mM ethylenediaminetetraacetic acid, pH 7–8) and frozen at −80 °C until analysis. Normalized DNA samples were thawed and denatured by heating for 10 minutes at 100 °C, cooled on ice for 5 minutes, and microfuged for 5 seconds. We then added 8.2 µL of 300 mM sodium acetate buffer (pH 5.2), 1.3 µL of 5 mM zinc chloride, and 7 µL of 25 units per milliliter Nuclease P1 to each sample. The tubes were inverted and microfuged for 5 seconds, and then incubated at 37 °C for 30 minutes. The samples were pH-adjusted to pH 7.5 to 8.0 by adding 14 µL 1 M tris(hydroxymethyl)aminomethane -hydrochloride (pH 7.5), and then 10.2 µL of 10 units per milliliter alkaline phosphatase was added to each sample. Samples were mixed by inversion, microfuged for 5 seconds and incubated at 37 °C for 30 minutes. The alkaline phosphatase was inactivated by boiling for 10 minutes at 95 °C, and the samples were then placed on ice. Aliquots (30 µL, 0.075 microgram per microliter) were stored at −20 °C until analysis. Concentrations of 8-OH-dG were determined by enzyme-linked immunosorbent assay using the DetectX DNA Damage Immunoassay Kit (Arbor Assays, Ann Arbor, Mich.) using a BMG FLUOstar Omega microplate reader (BMG LABTECH, Inc., Cary, N.C.); additional QC information is provided in appendix 2.
Examinations of Tissues for Microscopic Lesions
Formalin-fixed liver and kidney samples were dehydrated, paraffin-embedded, sectioned at 5 micrometers, and stained with hematoxylin and eosin, Fite’s acid fast, and Ziehl-Neelsen acid fast stains for light microscopic examination (Luna, 1968) at the USGS National Wildlife Health Center (Madison, Wisconsin). The tissues were examined for microscopic abnormalities potentially associated with lead toxicosis, including acid-fast intranuclear inclusions in renal tubular epithelial cells, hepatic or renal tubular degeneration and necrosis, hepatic or renal inflammation, hepatic lipidosis, hepatic hemosiderosis, hepatic bile stasis, hepatic biliary hyperplasia, hepatic or renal cyto/karyomegaly, and renal glomerulopathy (Haschek and others, 2013). Abnormalities were scored as present or absent by a single pathologist.
Monitoring Reproductive Success
Reproductive success was monitored in eastern bluebirds, eastern towhees, field sparrows, indigo buntings, and northern cardinals (table 2). Eastern bluebirds are a secondary cavity nesting species; therefore, wooden nest boxes were erected at each contaminated and reference site to facilitate efficient reproductive monitoring and tissue collection of bluebirds. Nest boxes were constructed using the eastern/western bluebird nest box design from the North American Bluebird Society (2016); we fitted the nest boxes with predator guards to reduce nest predation (for example, by cats, raccoons, and snakes). Each nest box was placed next to open fields with nearby perches and at least 50 m of spacing between boxes in all directions (Willner and others, 1983). The number of boxes at each site was dictated by the amount of suitable habitat (table 2).
Open-cup nests of eastern towhees, field sparrows, indigo buntings, and northern cardinals were located and monitored between early April and late July in 2016, 2017, and 2018 (table 2). We visited sites every 1 to 3 days to search for nests; nests were located using a combination of parental behavior and systematic searching (Ralph and others, 1993). Each nest was designated with a unique identification code and marked with weather-resistant flagging placed ≥5 meters (m) from the nest.
Nest boxes and open-cup nests were monitored using established protocols (Ralph and others, 1993). Nests were monitored every 1 to 7 days during the breeding season (May to July), depending on nest age or stage, until the nest fledged or failed; this interval ensured optimal timing for capture and tissue sampling of adults and their nestlings. The status of the nest and its contents (in other words, the number of eggs or nestlings of conspecific young and cowbirds) were recorded during each visit. Adult birds were targeted for capture during the nestling stage. Nestlings were hand-captured from the nest for sampling about 1 to 3 days before their predicted date of fledging (Ralph and others, 1993). Disturbances to the open-cup nests were limited by quickly completing checks with as little alteration to the surrounding vegetation as possible. Nests were not checked if common nest predators, such as Cyanocitta cristata (Linnaeus, 1758) (blue jays), Corvus brachyrhynchos (C. L. Brehm, 1822) (American crows), or brown-headed cowbirds (a brood parasite), were nearby.
Nest fate was determined using the expected fledge date in conjunction with observations made at subsequent visits or at the final check. Successful fledging was confirmed by observation of at least one nestling leaving the nest, or through a combination of other cues, including adults carrying food repeatedly to the same area (likely indicating they are feeding fledged birds away from the nest), begging calls, and trails of fecal sacs/feces leading away from the nest. Visual confirmations of fledglings were always attempted, but we were also cautious to minimize disturbances to adults and their broods. If no evidence of fledging was found, we monitored the territory for any immediate re-nesting attempts, which would suggest that a predation event had occurred. Nests were considered successful even if they only fledged cowbirds because this still indicated that a nest did not fail due to predation or lack of parental care. Nests with unknown final fates were included in survival analysis, but the final monitoring interval was omitted for nests for which fate was unknown.
At each open-cup nest, several habitat features that reflected the amount of vegetative cover around a nest were measured after the nest fledged or failed (Roach and others, 2018). These variables were assessed for their potential contributions to variation in reproductive success, in addition to local lead concentrations. Nest concealment was determined by averaging the visually estimated concealment of the nest from 1 m north, east, south, west, and above the nest. Percent ground cover was estimated by averaging the visually estimated percent vegetative ground cover in the northeast, southeast, southwest, and northwest quadrants of a circle around the nest defined by a 5-m radius; the mean percent shrub cover was similarly estimated. The number of saplings (2.5- to 12.5-centimeter [cm] diameter at breast height [DBH]), pole timber (13.0- to 27.5-cm DBH), and saw timber (>27.5-cm DBH) sized trees were tallied within an 11.3-m radius of the nest; counts were converted to number of stems per hectare in each diameter class.
Statistical Analyses and Data Treatments
Lead in Soils and Invertebrates
Soil results (pXRF) are presented as arithmetic means in cases where all results had detections, and as Kaplan-Meier means where results included ND values (Helsel, 2005). Historically, residues having concentrations censored as below the LOD or limit of quantification (LOQ) were often assigned values of one-half the LOD or LOQ, as appropriate for the analytical method, for statistical analyses. It is now considered more appropriate to assign values describing a range of possible concentrations to censored values (that is, 0 to the LOD or LOQ), as described in Helsel (2005). Thus, for soils, the Kaplan-Meier approach was used to calculate the extremes of the mean lead concentration using a near-zero value (5 ppm) and the instrument detection limit (11 ppm) for samples with concentrations of lead below the instrument detection limit. ND lead concentrations in soil were entered as 0 in models.
Invertebrate results (ICP–MS) are presented as arithmetic means and standard errors; statistical analyses of lead concentrations in invertebrates were not performed due to low sample numbers. Although we did not perform statistical analyses of the invertebrate results, we compared the results to thresholds derived by the EPA using food chain modeling based on avian ingestion of lead in soils and invertebrates.
As previously mentioned, the lead concentrations in soils across the contaminated sites were heterogeneous; therefore, birds nesting on these sites could potentially be exposed to a wide range of lead concentrations. To address this heterogeneity, exposures of the adults and their young to soil-borne lead were estimated as the mean lead concentration in soil within a 60-m radius (in other words, a raster grid) of each nest. This mean concentration was referred to as the “local lead concentration” and was used in analyses relating bird reproductive success to lead concentrations in soil. The area defined by a 60-m radius (1.1 hectare) falls within the range of the territory sizes of the five nesting species that were analyzed for reproductive success (tables 1, 2). We considered the “local lead concentrations” in soils to be reasonable approximations of exposure conditions because these species are territorial during the breeding season, and most feeding and other activity occurs within their territory.
We calculated the local lead concentrations by creating an inverse-distance-weighted (IDW) spatial interpolation (that is, concentration modeling based on the proximity and lead concentrations measured in nearby soil samples). Spatial interpolation has previously been used to estimate metals concentrations in contaminated soils (Aelion and others, 2008; Qiao and others, 2018). The IDW model for each site was created using the Spatial Analyst toolset in Esri ArcGIS Pro v2.5 (Esri, Redlands, Calif.) at a resolution of 10 m with a rectangular extent defined by a 60-m buffer around nest locations. The optimal value for the exponent in the inverse-distance-weighting, and the optimal number of neighboring point samples to use to calculate each cell value, was determined by cross-validation of rasters based on 5, 10, 15, 20, and 30 neighboring points in Esri ArcGIS Pro v2.5 (table 5). The cross-validation process leaves out one of the samples and subsequently compares the predicted concentration to the removed sample, repeating for all samples. The raster resulting in the lowest mean and root-mean-square errors based on the cross validation was selected for each site (table 5). The mean lead concentration of soil in a 60-m radius around each nest was calculated from the resulting rasters with the Spatial Analyst toolset in Esri ArcGIS Pro v2.5.
Table 5.
Parameters used in the inverse-distance-weighted spatial interpolation of lead concentrations in soil for each site and the resulting means and root-mean-square errors in the Southeast Missouri Lead Mining District.[Inverse-distance-weighted spatial interpolation was used to create a 10-meter resolution raster of lead concentrations in soil for each site]
Results from onsite and offsite pXRF methods were included in the interpolations to maximize the number of point samples, to provide more complete spatial coverage of soil concentrations, and to better account for the potential spatial heterogeneity in lead concentration across sites and the variations in sample densities around nests. This combined and interpolated dataset is likely to provide the best spatial representation of exposure to soil-borne lead experienced by birds across each site. At points where both onsite and offsite measurements were taken, the onsite measurement was used because we believed it best represented exposure to the birds eating and nesting at the sites due to temporal environmental conditions (for example, onsite changes in moisture, blowing dusts, and so on). Differences in results between onsite and offsite methods for the same sample were minimal (app. 2); comparisons of logarithmically transformed (log, base 10) concentrations of lead measured onsite and in duplicate offsite pXRF samples (based on 221 points for which onsite and offsite concentration measurements were made) indicated a mean percent difference of 5.5 percent and a 95-percent confidence interval of 4.7 to 6.1 percent.
Lead in Bird Tissues
Blood sample results from 2016, 2017, and 2018 were analyzed separately for adults and nestlings but not reported separately by year. All statistical analyses were performed on a dw basis; a value of 0 was substituted for concentrations below the LOQ for determinations of arithmetic mean and standard error. To determine if lead concentrations in blood varied among sites, we used a generalized linear model with species, site, and the interaction of species × site as fixed factors and log-transformed blood lead concentration as the response variable; Tukey’s honestly significant difference test (α=0.05) was used for comparisons after collection. Soil-borne “local” concentrations of lead were highly variable within each site; therefore, we also related species’ lead concentrations in blood to local lead concentrations in soil using a generalized linear mixed (GLM) model with log-transformed local lead concentration in soil and species as fixed factors and site as a random factor (that is, because factors other than lead concentrations in soil could differ by site). Lead concentrations in blood and soil were log-transformed when relating them to each other or to site for modeling because transformation improved the normality of these variables and the model fit based on examination of model residuals and Akaike information criterion values. Furthermore, it seemed reasonable to hypothesize that the additive effects of lead would, at some point, level off as lead concentrations in soil increased to extreme levels. However, nontransformed values of lead concentrations in blood were used when relating adult blood to brood blood concentrations because we hypothesized that this was a linear relation. Mourning doves and American robins were not included in the analysis of nonlethal blood samples because we did not perform nest-based monitoring of these species, or local lead concentrations in soil were not available; however, these species were included in comparisons of lead concentrations in blood to literature-based toxicity thresholds. Linear and mixed models relating lead concentrations in blood to lead concentrations by site, by species, and in soil were conducted with the linear models (lm) and linear mixed-effects models (lmer) functions in R (version 3.4.1; R Core Team, 2019) and Proc Mixed in SAS (version 9.4, SAS Institute, Cary, N.C.).
We examined the relation between log-transformed adult lead concentrations in liver and kidney to species, site, and the interaction of species × site using a linear model fit in SigmaPlot (version 14, Systat Software Inc., San Jose, Calif.); as before, Tukey’s honestly significant difference test (α=0.05) were used for comparisons after collection. Species-specific comparisons among sites were only possible for American robins and northern cardinals because these were the only two species that were sacrificed at all study sites and had sufficient sample sizes for statistical comparisons.
δALAD Activity
Statistical analyses were conducted for 51 δALAD blood samples (table 4). δALAD activity was tested for homogeneity of variance (Levene’s test) and normality (Shapiro-Wilk test, normal probability plot) using SAS (version 9.4, SAS Institute, Cary, N.C.). Although these data met homogeneity of variance and normality requirements for analysis of variance (ANOVA), the δALAD activities of two samples were below the LOD (app. 2, table 2.1). The LOD value was assigned to both of these samples, and then δALAD activity from all four lethally collected species (American robin, northern cardinal, mourning dove, brown-headed cowbird; table 4) from reference sites was compared with the activity values for all four species from the contaminated sites by the Wilcoxon nonparametric test. Comparison of combined reference sites to all contaminated sites was repeated only for northern cardinals; sample size was disparate among species and study sites (table 4), and inadequate (low power) for comparison of reference and contaminated sites for other species. A Kaplan-Meier mean was used to describe the central tendency of the contaminated sites using a near-zero value (0.01) and the LOD (13.99) for samples with activity less than the LOD.
Linear regression was used to compare the relation between blood δALAD activity and log-transformed tissue lead residues (that is, blood, kidney, and liver). For ND samples (that is, samples having lead results censored below the LOQ; blood=8 NDs; kidney=2 NDs; liver=7 NDs), we assigned the method LOQ (app. 2, table 2.1). We conducted regression analyses according to Helsel (2005) by using the LOQ for NDs and then repeating the analyses assigning a value of 0.001 for NDs. We examined all four species in one set of regression analyses and the subset of northern cardinal measurements in another set of regression analyses.
Oxidative Stress Indicators and DNA Damage
Data were analyzed to determine whether differences in the concentrations of measured biomarkers existed between species and site types (in other words, contaminated versus reference sites). As previously mentioned, comparisons were only made for American robins and northern cardinals because of low sample numbers for the other two species. We did not combine biomarker results from multiple species in the current analysis to not obscure differences in responses to lead exposure. R package MANOVA.RM (R version 3.4.3; R Core Team, 2019) was used to perform nonparametric inference for the comparison of the multivariate data samples. This test does not rely on specific distributional assumptions, covariance, or correlation structures, and is particularly suitable for datasets with small sample sizes (Friedrich and others, 2019). The permutation test included 1,000 replications, and a probability value (p) <0.05 was considered to indicate statistical significance. The modified ANOVA-type statistic was used for p-value computations. If the global hypothesis was rejected (signifying that there was an overall difference between species or site types), univariate analyses were used to identify which endpoints differed by species or site type. The resulting p-values were adjusted for multiple testing using the parametric bootstrap modified ANOVA-type statistic and the Benjamini-Hochberg False Discovery Rate-based correction. The relations between biomarkers and log-transformed lead concentrations in liver or kidney were assessed by linear regression analysis using the lm procedure in R (version 3.4.3; R Core Team, 2019).
Relation of Reproductive Success to Lead Concentrations in Blood and Soil
Eastern bluebird reproductive success was analyzed separately from the open-cup nesting species, but with similarly structured models, because of differences in monitoring protocols and interpretation of some response variables. Eastern bluebirds used known nest locations (nest boxes), which can be reliably monitored from nest initiation, whereas open-cup nests were found opportunistically between the nest building and nestling stage. Therefore, some open-cup nests are found in later stages of the nest cycle, and the exposure period for survival analyses was limited to only the days a nest was observed (Mayfield, 1961; Johnson, 1979; Dinsmore and others, 2002; Shaffer, 2004). Furthermore, the analysis of the number of young fledged for open-cup nests only included nests that fledged one or more young and represents the number of young fledged by successful nests because some nests likely failed before we found them (Mayfield, 1975) and therefore cannot be included in the analysis. The sample of eastern bluebird nests for number of young fledged included all nests because all nest boxes were monitored from initiation and represented the average number of young per nest attempt. Additionally, we were able to consider the relations between reproductive success of eastern bluebirds to lead concentrations in blood and soil, whereas we could only consider the relation of reproductive success of open-cup nesters to lead concentrations in soil because of a smaller number of blood samples for those species.
Linear regression analysis was used to compare the lead concentrations in the blood of eastern bluebird adults with those of their broods; all lead concentrations in blood were log-transformed before analysis. A similar analysis could not be conducted for open-cup nesters because the numbers of brood blood samples for each species were small. The lead concentration in the blood of the female was used as the default for the adult unless she was not sampled, in which case the male’s lead concentration in blood was used. As previously mentioned, we reasoned that the lead concentration in the blood of the female should be a better predictor of reproductive success (relative to the male) because she produces the eggs and provides more parental care than the male, but that in the absence of a female’s blood, the male could be used as a surrogate because the males also feed their young.
We used GLM models to determine the relation of lead concentrations in blood (BloodPb) and in “local” soil (SoilPb) on the response variable of interest, where SoilPb was the mean concentration of lead in soil within a 60-m radius of a nest. Models for open-cup nesters also included a species effect (Species) and a SoilPb × Species interaction because we hypothesized that each species would respond differently to SoilPb. The Species effect was represented by dummy variables coded as 0,1 for eastern towhees, indigo buntings, and northern cardinals (field sparrow was the reference category), which indicated when the three other species were 0. The interaction between SoilPb and Species represented how the effect of SoilPb differed for each species from the reference species, field sparrow. SoilPb represented the conditional effect of lead concentration in soil when Species was at the reference level (field sparrow). Therefore, we calculated the effect of lead concentration in soil on each species by summing the SoilPb effect and the interaction effect (SoilPb × Species) for that species of interest; this summed effect represents the slope for the effect of lead concentration in soil on each species and is reported as the SoilPb effect on the species of interest (Brambor and others, 2006). All models included random effects for site and year to account for the possibilities that (1) species responses may be more homogenous within a site or year, and (2) there may be factors that varied by site or year for which the fixed effects could not account but which could be controlled by random effects.
Four reproductive measures were analyzed: clutch size, hatching success, nest success, and number fledged. Clutch size and hatching success were based on the number of conspecific eggs and what proportion of them hatched; we excluded any nests parasitized by brown-headed cowbirds because they often remove host eggs. However, for determining nest success and the number of young fledged, we included nests with cowbird nestlings because we were interested in the ultimate fate of the nest and the ability of the parents to fledge young; therefore, we considered cowbirds as part of their brood at this stage.
Because of the nature of the different reproductive measures, we used different distributions in our GLM models to relate reproduction to lead concentrations. Clutch size and number of young fledged were modeled with a Poisson distribution and log link because these responses were counts of eggs or chicks. The proportion of eggs that hatched (hatching success; number of eggs hatched per total number of eggs) was modeled with a binomial distribution and logit link because it was a proportion. Daily nest survival was modeled with a Bernoulli distribution and a modified logit link function because daily survival rate is a probability that ranges from zero to one and is an exponential function of the length of each interval (Shaffer, 2004). If the model with SoilPb effects was supported, we plotted the predicted response across the range of lead concentrations in soil using the mean for the random site and year effects. Period survival rate (that is, the probability that a nest survives from initiation to fledging) was plotted, as opposed to daily nest survival, because the former is a more interpretable measure of reproductive performance (Shaffer and Thompson, 2007). Period nest survival was calculated as DSRx, where DSR was the daily survival rate and x was the number of days of the nest period from the laying of the first egg to fledging; x=37, 23, 26, 26, and 27 for eastern bluebird, field sparrow, eastern towhee, indigo bunting, and northern cardinal, respectively.
Models relating reproductive measures to lead concentrations in soil or blood were fit using Markov chain Monte Carlo (MCMC) analysis (using the JAGS package in R; jagsUI 1.5.0; R version 3.6.0; R Core Team, 2019). The MCMC analysis was selected because models that were fit using a maximum likelihood estimation approach would not converge or properly estimate the random effects; moreover, simulation techniques such as MCMC analysis are better at fitting complex models (Kéry and Schaub, 2012). Estimates were based on 3 chains of 100,000 iterations, a burn-in of 10,000 iterations, a thinning rate of 5, and a posterior sample of 54,000. Diffuse normal priors were used for the regression parameters, and diffuse uniform priors were used for the standard deviation parameters. Lead concentrations in blood and soil were log-transformed because this improved normality and model fit based on examining deviance information criteria (DIC) values. The log-transformed concentrations were then scaled by subtracting the mean and dividing by the standard deviation; this centered all variables on zero, converted the concentrations to units of standard deviations, and facilitated model convergence and comparison of results. We evaluated model convergence by checking for R-hat values of <1.1 for all model parameters and inspected trace plots to check that Markov chains were well mixed (Link and Barker, 2010). An important advantage of Bayesian analyses based on MCMC analysis is the ability to summarize the posterior distribution, which in our case represented the probability distribution for the effect of lead concentration on a reproductive response of interest. For each modeled response, we present the posterior mean values, standard deviations, and 95-percent credible intervals for the model parameters; these parameters are analogous to regression coefficients, standard errors, and 95-percent confidence intervals and their measures of uncertainty in more traditional frequentist statistical methods (that is, least-squares or maximum-likelihood estimation). An effect is sometimes considered statistically significant if the 95-percent credible interval for the posterior mean does not overlap zero, but this depends on an arbitrarily defined probability level, similar to p-values in frequentist methods. Therefore, we present the proportion of the posterior distribution with the same sign as the mean, which can be interpreted as the probability that the parameter had a negative or positive effect.
We also compared the DIC for the model with the appropriate lead effects (BloodPb or SoilPb for eastern bluebirds; SoilPb + Species × SoilPb for open-cup nesters) to a model without these fixed effects. Models with lower DIC are generally considered to have greater support. Therefore, if the model with these lead effects had (1) a lower DIC than the null model, and (2) the 95-percent credible interval for the effects did not overlap zero, then we interpreted this outcome as strong support for the effect of lead on the reproductive response. We plotted predicted reproductive responses as a function of BloodPb or SoilPb for models that had strong support for the effects of BloodPb or SoilPb.
Relation of Reproductive Success to Habitat Structure
Least square means, standard errors, and 95-percent confidence limits were estimated for each habitat variable by species for contaminated and reference sites with a mixed model that included Species, Contaminated (0 for reference sites, 1 for contaminated sites), a Species × Contaminated interaction as a fixed effect, and Site and Year as random effects. The potential contribution of differences in habitat across the range of local lead concentrations in soil to reproductive success was determined by evaluating support for adding each habitat variable to the GLM models, which were described above for determination of the relations of reproductive success to local lead concentration. If support was found for the effect of local lead concentration on a reproductive success measure, we evaluated the DIC value of that model with and without the addition of each habitat parameter, thereby addressing whether each habitat parameter explained significant variation in reproductive success when the latter was not explained by the local lead concentration.
Results
Lead in Soils and Invertebrates
We characterized lead concentrations in soil at over 1,000 separate points among the study sites. Lead concentrations in soil ranged from 24 to 35,967 ppm at Anschutz Mine, and from 79 to 19,100 ppm at Magmont Vent (fig. 2, table 6). The concentrations depended on the exact location of the sample point within the site, but the wide ranges of concentrations of soil-borne lead across these sites indicates heterogeneity and supports our “local lead concentration” approach for evaluation of reproductive metrics. The Big River floodplain site had a narrower range of lead concentrations in soil (21 to 3,070 ppm; fig. 2, table 6) relative to Anschutz Mine and Magmont Vent; this result was not unexpected and is consistent with the inundation of the low-lying areas within the Big River floodplain site by historical flood waters. Flood waters typically deposit fine sediments onto the floodplain surface as the waters recede; the soil sampled in areas that received historical flood waters had the greatest mean lead concentrations. Lead concentrations in soils decreased (to about 50 ppm upslope) as the sampling distance from the outer edge of the historically flooded areas increased (fig. 3).
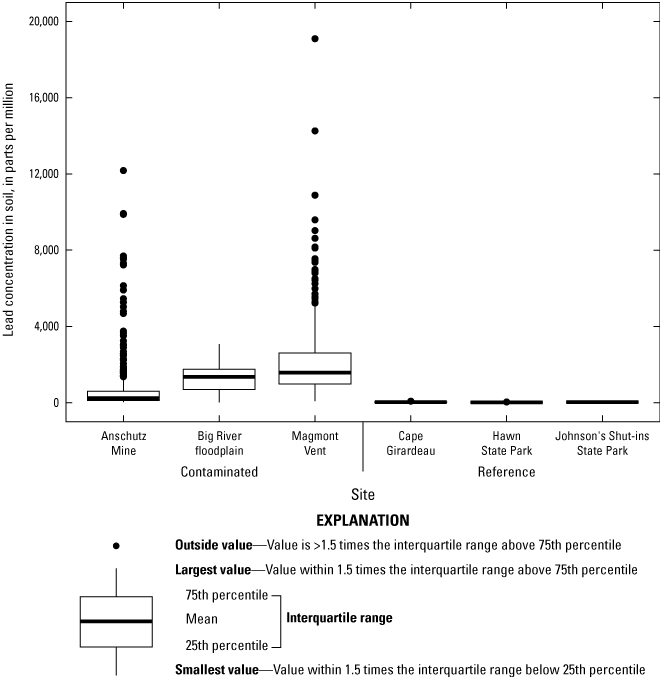
Lead concentration distributions in soils across the study sites, measured by onsite and offsite portable X-ray fluorescence spectrometry, in the Southeast Missouri Lead Mining District.
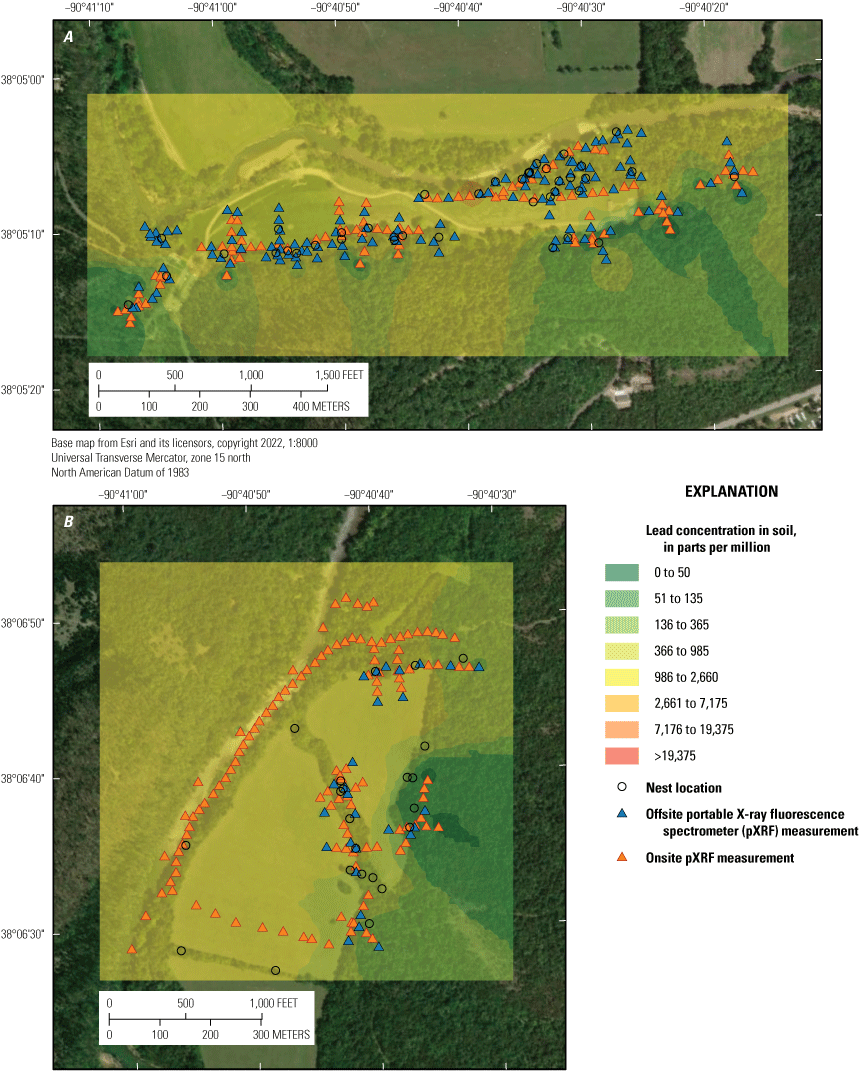
Spatially interpolated lead concentrations in soil at the contaminated Big River floodplain site in the Southeast Missouri Lead Mining District. A, a day use area in Washington State Park (Desoto, Missouri). B, a private tract of land along the Big River.
Table 6.
Lead concentrations in soils collected from contaminated and reference study sites in the Southeast Missouri Lead Mining District.[ND, not detected]
Lead concentrations in soil were measured using a portable X-ray fluorescence spectrometer (pXRF) using onsite and offsite measurement methods. The Kaplan-Meier method was used to present the extremes of the mean when one or more lead concentrations in the dataset were below the instrument limit of detection (app. 2, table 2.1). The near-zero value used for the Kaplan-Meier mean calculations was 5 parts per million (ppm); the instrument limit of detection was 11 ppm. Results are presented as mean (range) for all collection points at a given site, regardless of onsite or offsite measurement method. Soils analyzed onsite by pXRF were not dried and were measured as-is; soils analyzed using offsite pXRF were sieved and air-dried before analyses. Onsite analyses were not performed if the area of soil was too wet (that is, visible water or puddles were within 1 meter of the location). Typical background lead concentrations in Missouri soils range from 20 to 62 milligrams per kilogram (Beyer and others, 2013).
The greatest mean lead concentration in soil was at Magmont Vent (2,172 ppm; range 79 to 19,100 ppm; table 6). Anschutz Mine had the greatest discrete lead concentration (35,943 ppm) despite having the lowest mean concentration (852 ppm) of the contaminated sites. The mean lead concentration in soil at Big River floodplain was 1,292 ppm. The Hawn State Park and Johnson’s Shut-ins State Park reference sites soils had relatively low lead concentrations (<38 ppm; table 6), and lead concentrations in soil at the Cape Girardeau reference site were 17 to 99 ppm (table 6). Mean reference site lead concentrations were 21, 31, and 36 ppm at Hawn State Park, Johnson’s Shut-ins State Park, and Cape Girardeau, respectively. We did not evaluate the soil results for statistical differences among means at the sites because of heterogeneity. The heterogeneity of lead in the soils at each site is IDW-modeled in figure 3 (Big River floodplain), figure 4 (Magmont Vent), figure 5 (Anschutz Mine), and figure 6 (reference sites); error rates in the interpolation were low to moderate (table 5), but areas of the map far from the point sample locations could have substantial uncertainty. Maps based on the interpolations demonstrated that the reference sites were spatially homogenous and had relatively low lead concentrations (fig. 6), while the contaminated sites had a greater degree of spatial heterogeneity and locally elevated lead concentrations in soil (fig. 3, fig. 4, and fig. 5).
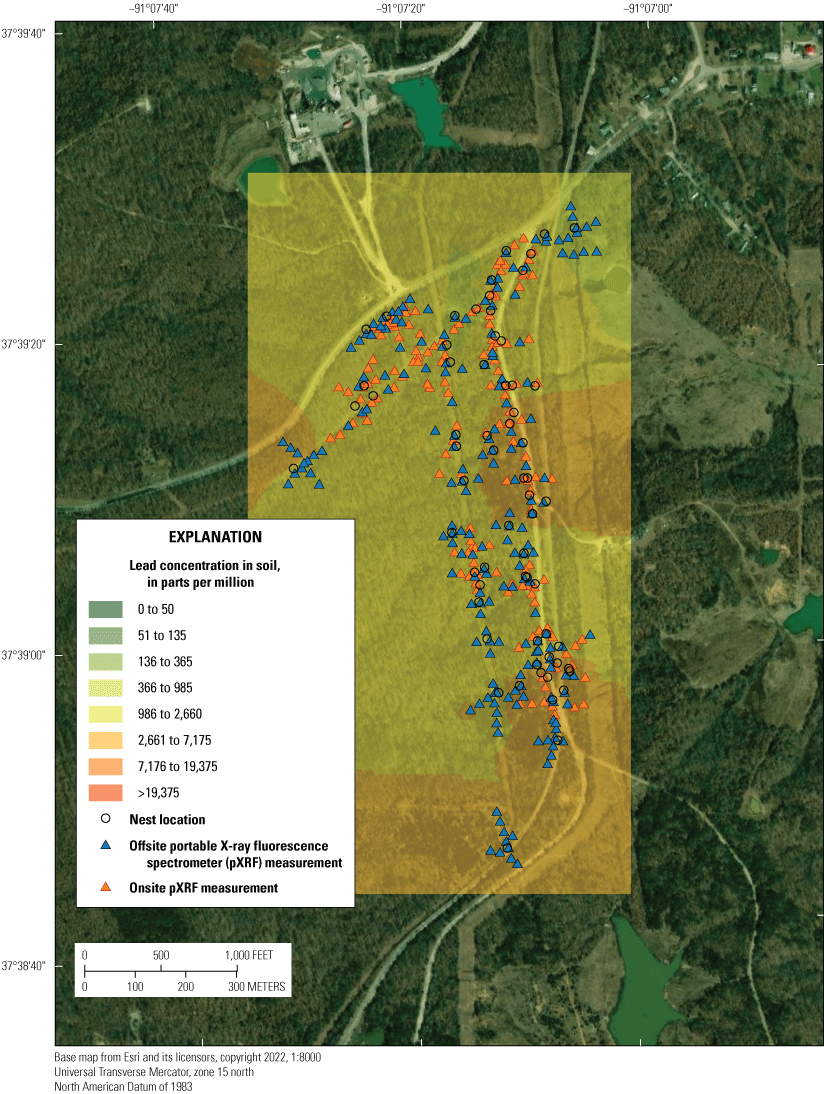
Spatially interpolated lead concentrations in soil at the contaminated Magmont Vent site (Bixby, Missouri) in the Southeast Missouri Lead Mining District.
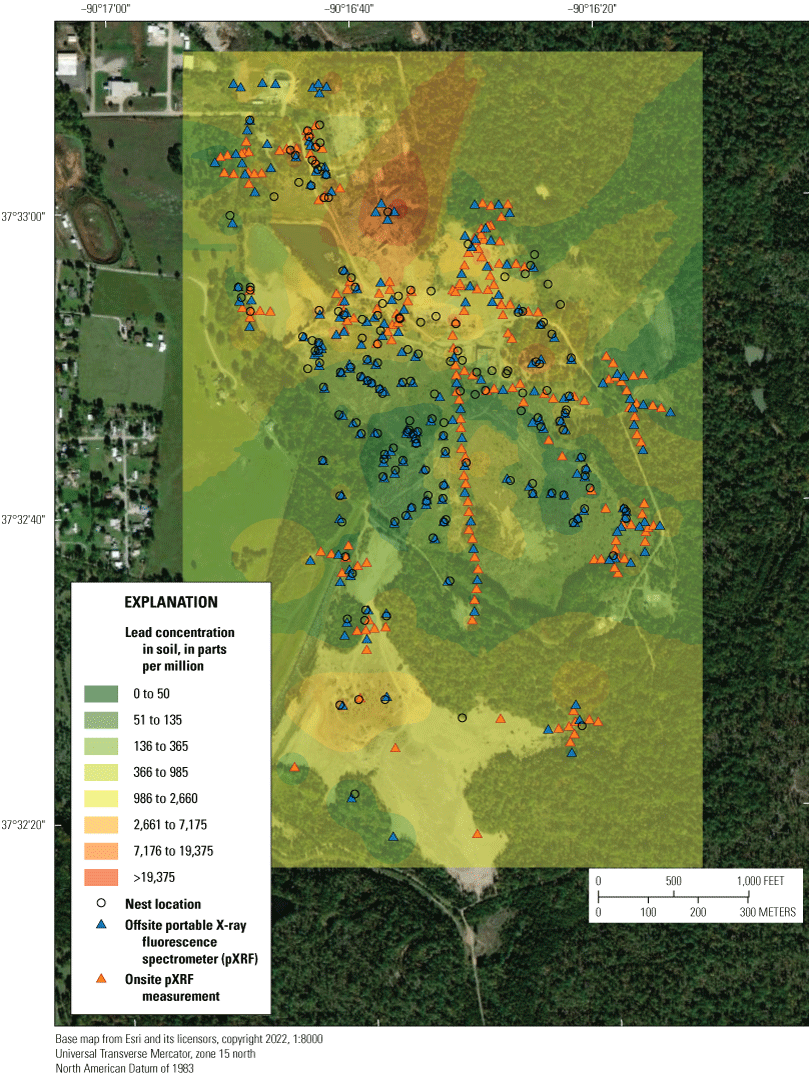
Spatially interpolated lead concentrations in soil at the contaminated Anschutz Mine site (Fredericktown, Missouri) in the Southeast Missouri Lead Mining District.
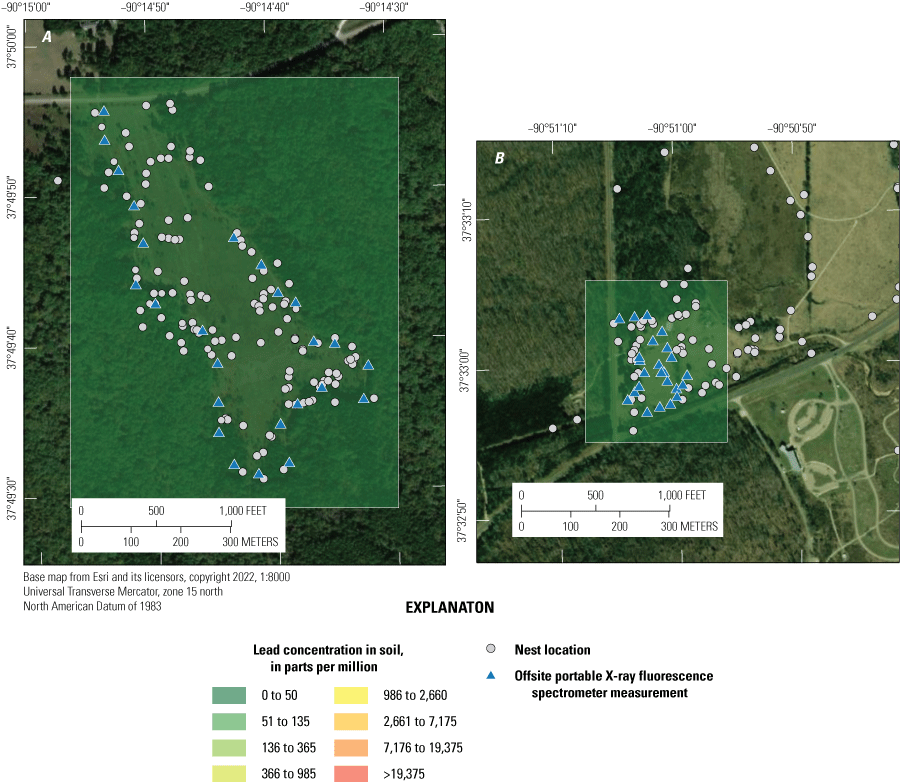
Spatially interpolated lead concentrations in soil at the reference sites in the Southeast Missouri Lead Mining District. A, Hawn State Park (Ste. Genevieve County, Missouri). B, Johnson’s Shut-ins State Park (Reynolds County, Mo.).
Lead concentrations in invertebrates varied by site but were generally lowest at the reference sites and greatest at the contaminated sites (table 7). Mean concentrations ranged from 9.63 to 15.0 mg/kg dw lead at the reference sites and from 397 to 1,171 mg/kg dw at the contaminated sites. However, invertebrate sample sizes were low (n=23 total samples) and collection was performed in 2016 only; invertebrate availability may vary by season and year, which may limit the representativeness of the 2016 sample results across the 3-year duration of the study.
Table 7.
Lead concentrations in mixed invertebrate samples collected near nesting areas at contaminated and reference sites in the Southeast Missouri Lead Mining District.[Data are summarized from Cleveland and others (2023). ±, plus or minus]
Samples are composited earthworms and beetle larvae that were not depurated; nor were samples rinsed to remove surficial soils and dusts. Composites consisted of about 90 percent earthworms and 10 percent beetle larvae by wet weight.
Lead concentrations were measured using inductively coupled plasma-mass spectrometry (ICP–MS) after microwave-assisted acid digestion and are reported in milligrams of lead per kilogram of lyophilized invertebrate tissue on a dry-weight basis as mean, standard error, and range. No results were below the limit of quantification for ICP–MS (app. 2, table 2.1). Statistical comparisons were not made due to low sample size.
Lead in Bird Tissues
Blood samples from 328 adult birds and 97 broods (composites, table 4) were analyzed for lead. Adult American robin, brown-headed cowbird, eastern bluebird, eastern towhee, field sparrow, indigo bunting, northern cardinal, and mourning dove were targeted for blood collection; however, not all target species were abundant at all sites. Anschutz Mine was the only site for which blood could be collected from all eight species; and only eastern bluebirds, indigo buntings, and northern cardinals were available at all sites (table 8, fig. 7). Based on the generalized linear model with species, site, and the interaction of Species × Site as fixed factors, lead concentrations in blood in birds from contaminated sites were, on average, 10 times greater than in birds from reference sites (fig. 7). Site (F5,288=83.15, p<0.001) and Species (F7,288=6.38, p<0.001) had significant effects on adult lead concentrations in blood. However, the interaction between Site and Species was not significant (F18,288=0.99, p=0.472), indicating that the effect of site was similar across species.
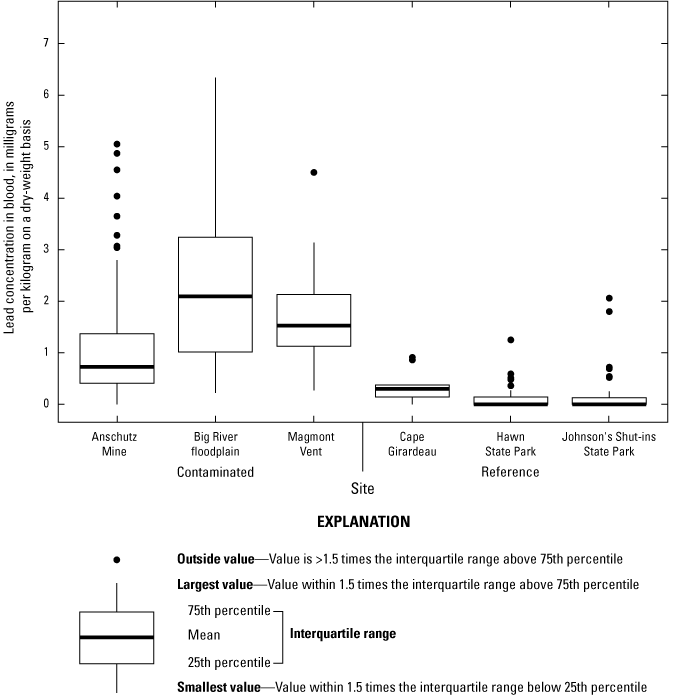
Distributions of the lead concentrations in the blood of adult songbirds breeding at the contaminated and reference sites in the Southeast Missouri Lead Mining District.
Table 8.
Lead concentrations in blood from adult birds and their broods at contaminated and reference sites in the Southeast Missouri Lead Mining District.[Data are summarized from Cleveland and others (2023). The results include blood samples collected via both nonlethal and lethal sampling approaches; and results for both male and female birds are included in the adult mean concentration values because both sexes feed their young. Adult blood samples were collected from individuals of the age class any hatch year (AHY); brood blood samples were composited by nest. A value of 0 was substituted for values censored as below the estimated limit of quantification (app. 2, table 2.1) in the mean and standard error calculations. n, number of samples; mg/kg dw, milligram per kilogram on a dry-weight basis; ±, plus or minus; --, no data; <, less than]
Inter-site comparisons were difficult to tease apart because lead concentrations in blood demonstrated a fair amount of variation within each site for each species (table 8). For example, the mean lead concentration in blood from adult indigo buntings did not differ among sites (p>0.05) in post-hoc comparisons; this was likely a result of relatively high variation in blood lead concentrations among individuals within sites (that is, the relative standard deviations for lead concentrations in blood samples ranged from 13.5 to 63.4 percent for adults and from 23.8 to 60.0 percent for broods). Eastern bluebirds at the Big River floodplain site had greater lead concentrations in blood than eastern bluebirds at Anschutz Mine (p<0.001) and the Hawn State Park and the Johnson’s Shut-ins State Park reference sites (p<0.001). Northern cardinals showed a similar pattern; the mean lead concentration in blood was greatest at Big River floodplain compared to Anschutz Mine (p<0.001) and the reference sites (p<0.001).
Based on the GLM model with log-transformed local lead concentration in soil (IDW-modeled) and species as fixed factors and site as a random factor, lead concentrations in blood generally increased with increasing local lead concentrations in soil for all species (adults and broods). Both lead concentrations in soil (SoilPb; F1,180=29.11, p<0.001) and Species (F4,18 =3.46, p=0.010) had effects on the lead concentration in blood of adult songbirds. However, the interaction between species and soil lead concentration was not significant (F4,180=1.23, p=0.298), indicating that the effect of lead concentration in soil on lead concentration in blood did not vary among species (fig. 8).
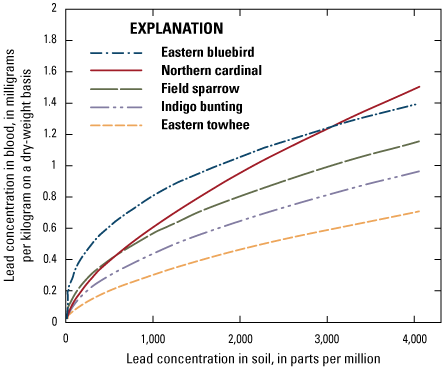
Relations between lead concentration in the soil in the breeding territory and lead concentration in blood for five songbird species at three contaminated sites (Anschutz Mine, Big River floodplain, and Magmont Vent) and two reference sites (Johnson’s Shut-ins State Park and Hawn State Park) in the Southeast Missouri Lead Mining District.
A similar trend was found with nestlings; there was a significant effect of lead concentration in soil (F1,78=55.07, p<0.001) and a marginal effect of species (F4,78=2.45, p=0.053) on lead concentration in nestling blood. The interaction of lead concentration in soil and species was not significant (F4,78=1.98, p=0.105), indicating that the effect of lead concentration in soil did not vary among species. The sample size of paired adult and nestling lead concentrations in blood for eastern bluebirds (n=44 nests) permitted examination of the relation between exposure in adults and their broods. There was a strong, predictive relation between the lead concentration in blood in adult eastern bluebirds and the mean lead concentration in the blood of their broods (F1,42=88.76, R2=0.67, p<0.001; fig. 9). In other words, adults with greater lead concentrations in blood had broods with greater lead concentrations in blood.
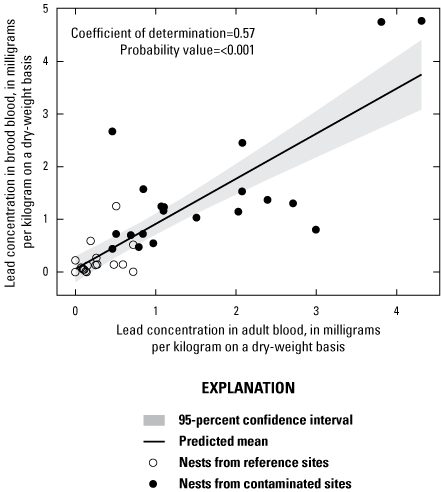
Relation between the lead concentration in the blood adult Sialia sialis (Linnaeus, 1758) (eastern bluebird) with the lead concentration in the blood of their broods based on a linear regression model.
Overall, lead concentrations in kidneys were generally greater at the contaminated sites compared to the reference sites; however, exact differences were species-specific (table 9; fig. 10). On average, lead concentrations in kidneys of birds from the contaminated sites (n=67) were 8 times greater than the lead concentrations in kidneys of birds from reference sites (n=20). There was an effect of species (F3,86=12.687, p<0.001), site (F5,86=30.936, p<0.001), and an interaction between species and site (F4,86=3.164, p=0.019) on lead concentrations in adult kidneys (table 10). We also found effects of site when analyzing kidneys of American robins (F2,34=15.051, p<0.001; table 11) and northern cardinals (F4,32=30.594, p<0.001; table 12); kidneys from individuals at contaminated sites had greater lead concentrations in their kidneys relative to those at the reference sites. The mean lead concentration in kidneys of northern cardinals at Big River floodplain (18.4 mg/kg dw) was greater than the mean lead concentrations in kidneys at all other sites (0.26 to 2.80 mg/kg dw; tables 9 and 13); the northern cardinals sampled at Johnson’s Shut-ins State Park had the lowest mean lead concentration in kidneys (0.26 mg/kg dw).
Table 9.
Lead concentrations in livers and kidneys from adult birds at contaminated and reference sites in the Southeast Missouri Lead Mining District.[Data are summarized from Cleveland and others (2023). Both male and female birds were sampled because both sexes feed their young. A value of 0 was substituted for values censored as below the estimated limit of quantification in the mean and standard error calculations. n, number of samples; mg/kg dw, milligram per kilogram on a dry-weight basis; ±, plus or minus; --, no data; <, less than]
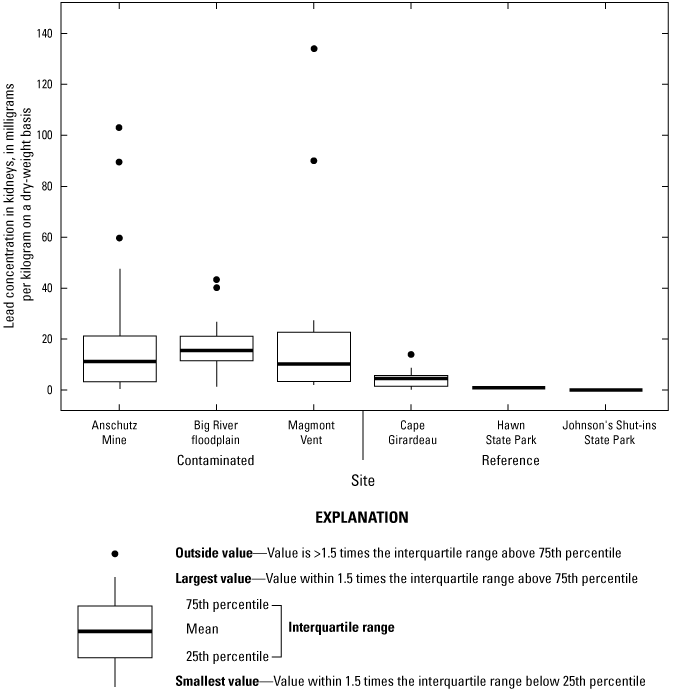
Distributions of lead concentrations in kidneys of adult songbirds by site for all species combined (Turdus migratorius [Linnaeus, 1766] [American robin], Molothrus ater [Boddaert, 1783] [brown-headed cowbird], Zenaida macroura [Linnaeus, 1758] [mourning dove], and Cardinalis cardinalis [Linnaeus, 1758] [northern cardinal]) in the Southeast Missouri Lead Mining District.
Table 10.
Output of the generalized linear model examining the effects of species and site on lead concentrations in kidneys in the Southeast Missouri Lead Mining District.[Lead concentrations in kidneys were logarithmically (base 10)-transformed for modeling. SS (Type III), Sum of Squares with the Type III method applied; df, degree of freedom; F, F value; p, probability value; --, no data]
Table 11.
Output of the generalized linear model examining the effect of site on lead concentrations in kidneys of Turdus migratorius (Linnaeus, 1766) (American robin) in the Southeast Missouri Lead Mining District.[Lead concentrations in kidneys were logarithmically (base 10)-transformed for modeling. SS (Type III), Sum of Squares with the Type III method applied; df, degree of freedom; F, F value; p, probability value; --, no data]
Table 12.
Output of the generalized linear model examining the effect of site on lead concentrations in kidneys of Cardinalis cardinalis (Linnaeus, 1758) (northern cardinal) in the Southeast Missouri Lead Mining District.[Lead concentrations in kidneys were logarithmically (base 10)-transformed for modeling. SS (Type III), Sum of Squares with the Type III method applied; df, degree of freedom; F, F value; p, probability value; --, no data]
Table 13.
Tukey’s post-hoc comparisons of means of homogenous subsets of lead concentrations in kidneys of Cardinalis cardinalis (Linnaeus, 1758) (northern cardinal) by site in the Southeast Missouri Lead Mining District.[Lead concentrations in kidneys were logarithmically (base 10)-transformed for modeling. Homogeneous subset tables indicate groups having the same or different means. --, no data; p, probability value]
Similarly, mean lead concentrations in liver tissues were greater at the contaminated sites compared to the reference sites (fig. 11; table 9); there was a significant effect of site (F5,86=15.65, p<0.001) and an interaction between species and site (F4,86=2.70, p=0.037) (table 14). However, species alone had no effect (F3,86=1.796, p=0.155; table 14). There was also an effect of site (F4,32=13.866, p<0.001) when examining lead concentrations in livers of northern cardinals only (table 15). The mean lead concentration in livers of northern cardinals was greatest at the Big River floodplain (12.1 mg/kg dw), followed by Magmont Vent (2.55 mg/kg dw), the Anschutz Mine (1.97 mg/kg dw), Hawn State Park (0.85 mg/kg dw), and, finally, Johnson’s Shut-ins State Park (0.26 mg/kg dw; table 9) sites. However, there were no significant differences between lead concentrations in livers of northern cardinals between Hawn State Park and Johnson’s Shut-ins State Park, nor were there differences between lead concentrations in liver at Anschutz Mine and Magmont Vent (table 16). We also found site effects for lead concentrations in livers of American robins (F2,34=20.349, p<0.001; table 17); mean lead concentrations in liver were greatest at Anschutz Mine (7.62 mg/kg dw) and least at the Cape Girardeau reference site (0.96 mg/kg dw; table 9).
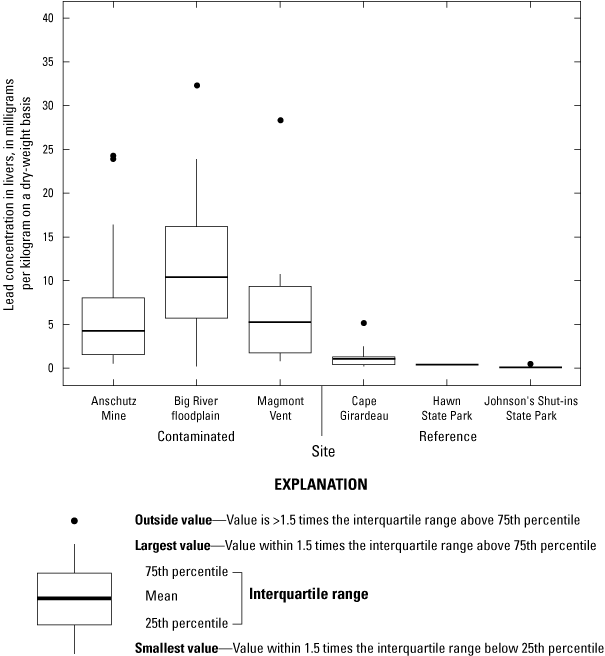
Distributions of lead concentrations in livers of adult songbirds by site for all species combined (Turdus migratorius [Linnaeus, 1766] [American robin], Molothrus ater [Boddaert, 1783] [brown-headed cowbird], Zenaida macroura [Linnaeus, 1758] [mourning dove], and Cardinalis cardinalis [Linnaeus, 1758] [northern cardinal]) in the Southeast Missouri Lead Mining District.
Table 14.
Output of the generalized linear model examining the effects of species and site on lead concentrations in livers in the Southeast Missouri Lead Mining District.[Lead concentrations in livers were logarithmically (base 10)-transformed for modeling. SS (Type III), Sum of Squares with the Type III method applied; df, degrees of freedom; F, F value; p, probability value; --, no data]
Table 15.
Output of the generalized linear model examining the effect of site on concentrations of lead in livers of Cardinalis cardinalis (Linnaeus, 1758) (northern cardinal) in the Southeast Missouri Lead Mining District.[Lead concentrations in livers were logarithmically (base 10)-transformed for modeling. SS (Type III), Sum of Squares with the Type III method applied; df, degrees of freedom; F, F value; p, probability value; --, no data]
Table 16.
Tukey’s post-hoc comparisons of means of homogenous subsets of concentrations of lead in livers of Cardinalis cardinalis (Linnaeus, 1758) (northern cardinal) by site in the Southeast Missouri Lead Mining District.[Lead concentrations in kidneys were logarithmically (base 10)-transformed for modeling. Homogeneous subsets tables indicate groups having the same or different means. --, no data; p, probability value]
Table 17.
Output of the generalized linear model examining the effect of site on lead concentrations in livers of Turdus migratorius (Linnaeus, 1766) (American robin) in the Southeast Missouri Lead Mining District.[Lead concentrations in livers were logarithmically (base 10)-transformed for modeling. SS (Type III), Sum of Squares with the Type III method applied; df, degrees of freedom; F, F value; p, probability value; --, no data]
δALAD Activity
When averaging values across all species, blood δALAD activity in samples from reference sites (overall mean of 190.3 activity units) was greater (Wilcoxon non-parametric test, Z=4.3738, χ2=19.2158, p<0.001) than activity in birds from contaminated sites (Kaplan-Meier mean of 88.4 to 89.3; table 18). Two of 19 individuals from the reference sites had δALAD activity values <50 percent (that is, <95.2 activity units) of the overall mean (190.3 units) activity for the reference sites, whereas 18 of 34 (53 percent) individuals at contaminated sites had δALAD activity values <50 percent of the overall mean activity (190.3 units) of those at the reference sites. Likewise, δALAD activity of northern cardinals (n=25 total animals) at the reference sites (mean=204.2 activity units) was greater (Wilcoxon non-parametric test, Z=3.2559, χ2=10.7862, p=0.001) than activity of cardinals from the contaminated sites (Kaplan-Meier mean of 88.4 to 90.1; table 18). None of the northern cardinals from the reference sites had activity <50 percent (that is, <102.1 activity units) of the mean activity of cardinals from reference sites, whereas 8 of 16 (50 percent) of the northern cardinals from the contaminated sites had δALAD activity <50 percent of the mean for cardinals from reference sites.
Table 18.
Mean δ-aminolevulinic acid dehydratase activities of avian blood samples collected at reference and contaminated sites in the Southeast Missouri Lead Mining District.[Data are summarized from Cleveland and others (2023). Comparisons of δALAD activity were made between reference and contaminated sites using the Wilcoxon non-parametric test for all avian species combined and for the subset of Cardinalis cardinalis (Linnaeus, 1758) (northern cardinals). Comparisons of reference and contaminated sites for just northern cardinal samples were possible because several individuals of this species were collected. <, less than; LOD, limit of detection; δALAD, δ-aminolevulinic acid dehydratase]
The four species having δALAD analyses were Turdus migratorius (Linnaeus, 1766) (American robin), northern cardinal, Zenaida macroura (Linnaeus, 1758) (mourning dove), and Molothrus ater (Boddaert, 1783) (brown-headed cowbird).
Using samples from 51 individuals (all 4 species combined), regression ANOVA indicated a relation (p<0.001) between blood δALAD activity (NDs assigned LOD) and log-transformed lead concentration in blood (F1,49=57.8 for NDs assigned 0.001 as a default value; F1,49=113.8 for NDs assigned LOQ as a default value), kidney (F1,49=55.7 for NDs assigned 0.001; F1,49=94.5 for NDs assigned LOQ) and liver (F1,49=20.0 for NDs assigned 0.001; F1,49=40.5 for NDs assigned LOQ) tissues. Visual inspection of blood δALAD and tissue lead plots (fig. 12) indicated an inverse relation for activity and lead concentration. Adjusted coefficient of determination (R2) values for these relations ranged from 0.28 to 0.69 (table 19).
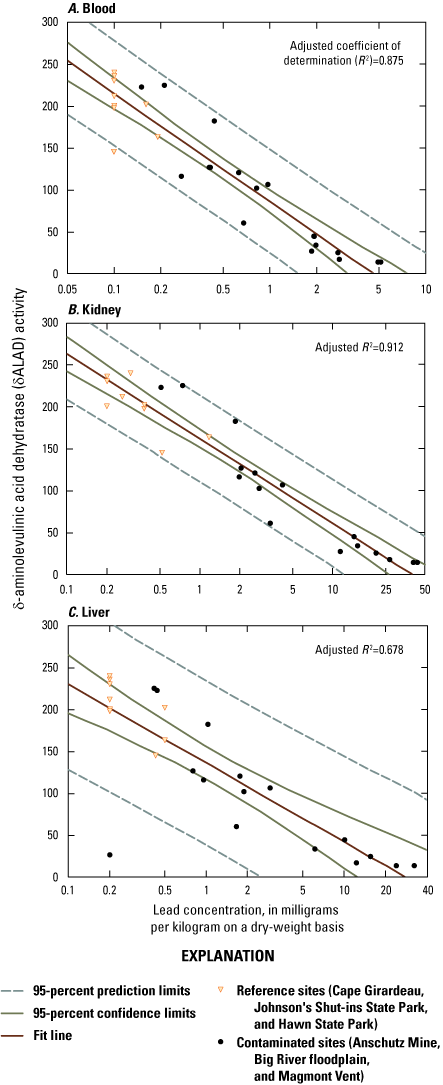
Relation of δ-aminolevulinic acid dehydratase (δALAD) activity and lead concentrations in tissues of Cardinalis cardinalis (Linnaeus, 1758) (northern cardinal) in the Southeast Missouri Lead Mining District. A, blood. B, kidneys. C, livers.
Table 19.
Relation between δ-aminolevulinic acid dehydratase activity and logarithmically (base 10) transformed lead concentrations in blood, kidneys, and livers in the Southeast Missouri Lead Mining District.[Data are summarized from Cleveland and others (2023). Adjusted coefficient of determination (R2) values of the relation between δ-aminolevulinic acid dehydratase (δALAD) activity and logarithmically (base 10)-transformed lead tissue residues using values of the limit of quantification (LOQ) and the near zero value of 0.001. The estimated LOQ was used as the lead concentration for samples having lead concentrations below the LOQ. δALAD activities were measured on a wet-weight tissue basis; lead concentrations in blood, kidneys, and livers were measured on a dry-weight basis. The LOQ for lead in blood was 0.1 milligram per kilogram on a dry-weight basis (mg/kg dw); the LOQ for lead in kidneys and livers was 0.2 mg/kg dw. The four species having δALAD analyses were Turdus migratorius (Linnaeus, 1766) (American robin), Cardinalis cardinalis (Linnaeus, 1758) (northern cardinal), Zenaida macroura (Linnaeus, 1758) (mourning dove), and Molothrus ater (Boddaert, 1783) (brown-headed cowbird). <, less than]
When only the δALAD results for northern cardinals were considered, regression ANOVA demonstrated a similar relation (p<0.001) between blood δALAD activity (NDs assigned LOD) and log-transformed lead concentrations in blood (F1,23=51.1 for NDs assigned 0.001 as a default value; F1,23=169.1 for NDs assigned LOQ as a default value), kidney (F1,23=52.4 for NDs assigned 0.001; F1,23=248.8 for NDs assigned LOQ), and liver (F1,23=18.2 for NDs assigned 0.001; F1,23=51.6 for NDs assigned LOQ) tissues. The adjusted R2 values for these relations in northern cardinals ranged from 0.417 to 0.912 and were seemingly more robust (that is, greater R2 values) than the relations for all four species combined (table 19).
Oxidative Stress Indicators and DNA Damage
Statistical analyses of species and site-type differences in the targeted endpoints (biochemical markers TBARS, GSH, tGSH, GSSG, GSSG:GSH, TSH, PBSH, 8-OH-dG) were limited to northern cardinals and American robins. Analyses could not be performed on data for mourning doves and brown-headed cowbirds due to small sample size (n=1 each). For American robins and cardinals, we analyzed the effects of species, site type (reference versus contaminated sites), and their interaction. We found effects of both factors (species M=195.430, p<0.001; site-type M=51.003, p=0.007) and a marginal interaction (M=25.796, p=0.069). Differences between species (from contaminated versus reference sites) were observed for GSH (p<0.001), tGSH (p=0.007), GSSG (p=0.002), GSSG:GSH ratio (p<0.001), TSH (p<0.001), PBSH (p<0.001), and TBARS (p=0.005) (fig. 13), but not for DNA damage (not shown).
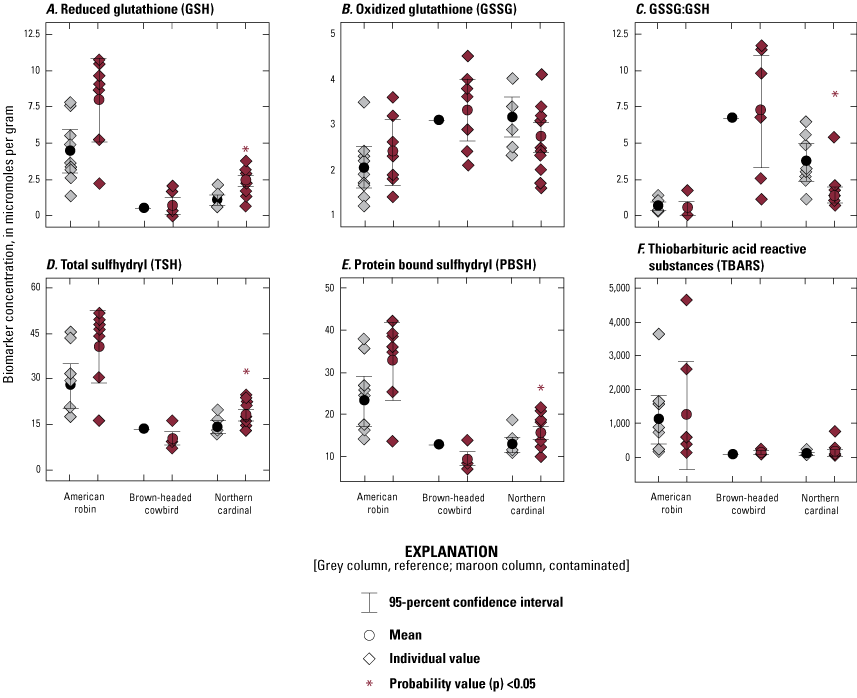
Concentrations of oxidative stress markers in the livers of adult songbirds. A, reduced glutathione (GSH). B, oxidized glutathione (GSSG). C, the ratio of GSSG to GSH (GSSG:GSH). D, total sulfhydryl (TSH). E, protein bound sulfhydryl (PBSH). F, thiobarbituric acid reactive substances (TBARS).
Data for site-type effects were analyzed separately for American robins and northern cardinals (table 20). No differences between site type (contaminated versus reference) were observed for American robins for any endpoints. For northern cardinals, concentrations of GSH (p=0.047), GSSG:GSH ratio (p=0.032), TSH (p=0.032), and PBSH (p=0.047) differed between contaminated and reference samples. Concentrations of GSH, TSH, and PBSH were greater in northern cardinals from contaminated sites compared to reference cardinals, whereas the GSSG:GSH ratio was greater in reference northern cardinals compared to northern cardinals from lead-contaminated sites (table 20). No differences were observed in TBARS or levels of DNA damage (8-OH-dG) between reference and contaminated samples.
Table 20.
Oxidative stress and deoxyribonucleic acid damage indicators in liver samples collected from Turdus migratorius (Linnaeus, 1766) (American robin) and Cardinalis cardinalis (Linnaeus, 1758) (northern cardinal) at reference and contaminated sites in the Southeast Missouri Lead Mining District.[Data are summarized from Cleveland and others (2023). Concentrations were measured on a wet-weight tissue basis. Results for Molothrus ater (Boddaert, 1783) (brown-headed cowbird) and Zenaida macroura (Linnaeus, 1758) (mourning dove) are not shown due to low sample numbers. ±, plus or minus; n, number of samples; TBARS, thiobarbituric acid reactive substances; GSH, reduced glutathione; tGSH, total glutathione; GSSG, oxidized glutathione; TSH, total sulfhydryl; PBSH, protein bound sulfhydryl; 8-OH-dG, 8-hydroxy-2'-deoxyguanosine; µmol/g, micromole of analyte per gram of sample; ng/mL, nanograms of analyte per milliliter of sample]
A positive relation was observed in American robins between log-transformed lead concentrations in liver and GSH (F1,15=5.11, p=0.039) and tGSH (F1,15=8.758, p=0.010) and between log-transformed lead concentrations in kidneys and tGSH (F1,15=8.182, p=0.012) (fig. 14). In northern cardinals, we observed positive relations between log-transformed lead concentrations in kidneys and GSH (F1,24=11.45, p=0.002), tGSH (F1,24=3.653, p=0.068), TSH (F1,24=6.29, p=0.019), and PBSH (ρ=0.445, p=0.023); there was a negative relation between lead concentrations in kidneys and the log-transformed ratio of GSSG:GSH (F1,24=7.393, p=0.012) (fig. 15). Similarly, we observed positive relations in northern cardinals between log-transformed lead concentrations in livers and GSH (F1,24=12.2, p=0.002), TSH (F1,24=7.634, p=0.011), and PBSH (F1,24=5.279, p=0.030); there was a negative relation between lead concentrations in livers and the log-transformed GSSG:GSH ratio (F1,24=8.52, p=0.008) (fig. 16). In brown-headed cowbirds, no relations were observed between lead concentrations in livers or kidneys and any of the monitored endpoints. No relations between 8-OH-dG or TBARS with lead concentrations in either liver or kidney tissues were found in any species.
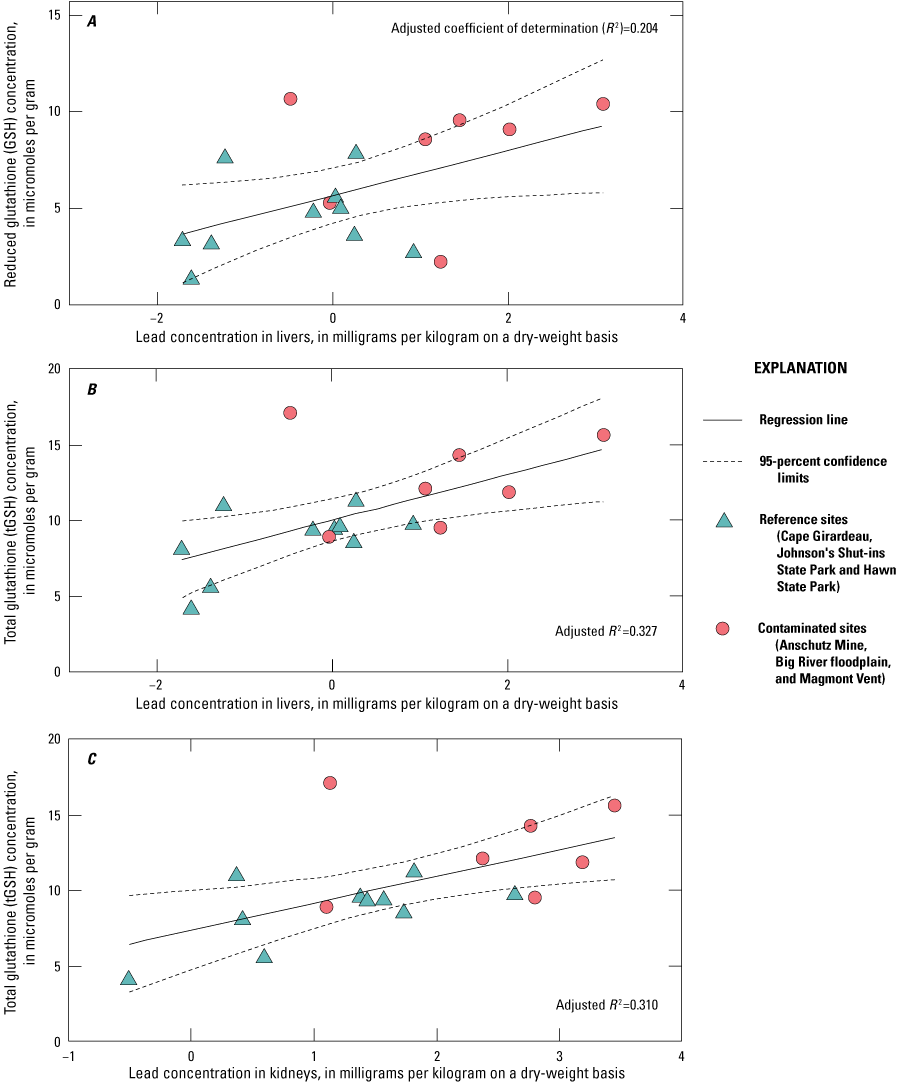
Relations between oxidative stress markers and lead concentrations in livers and kidneys of Turdus migratorius (Linnaeus, 1766) (American robin) in the Southeast Missouri Lead Mining District. A, reduced glutathione (GSH) to livers. B, total glutathione (tGSH) to livers. C, tGSH to kidneys.
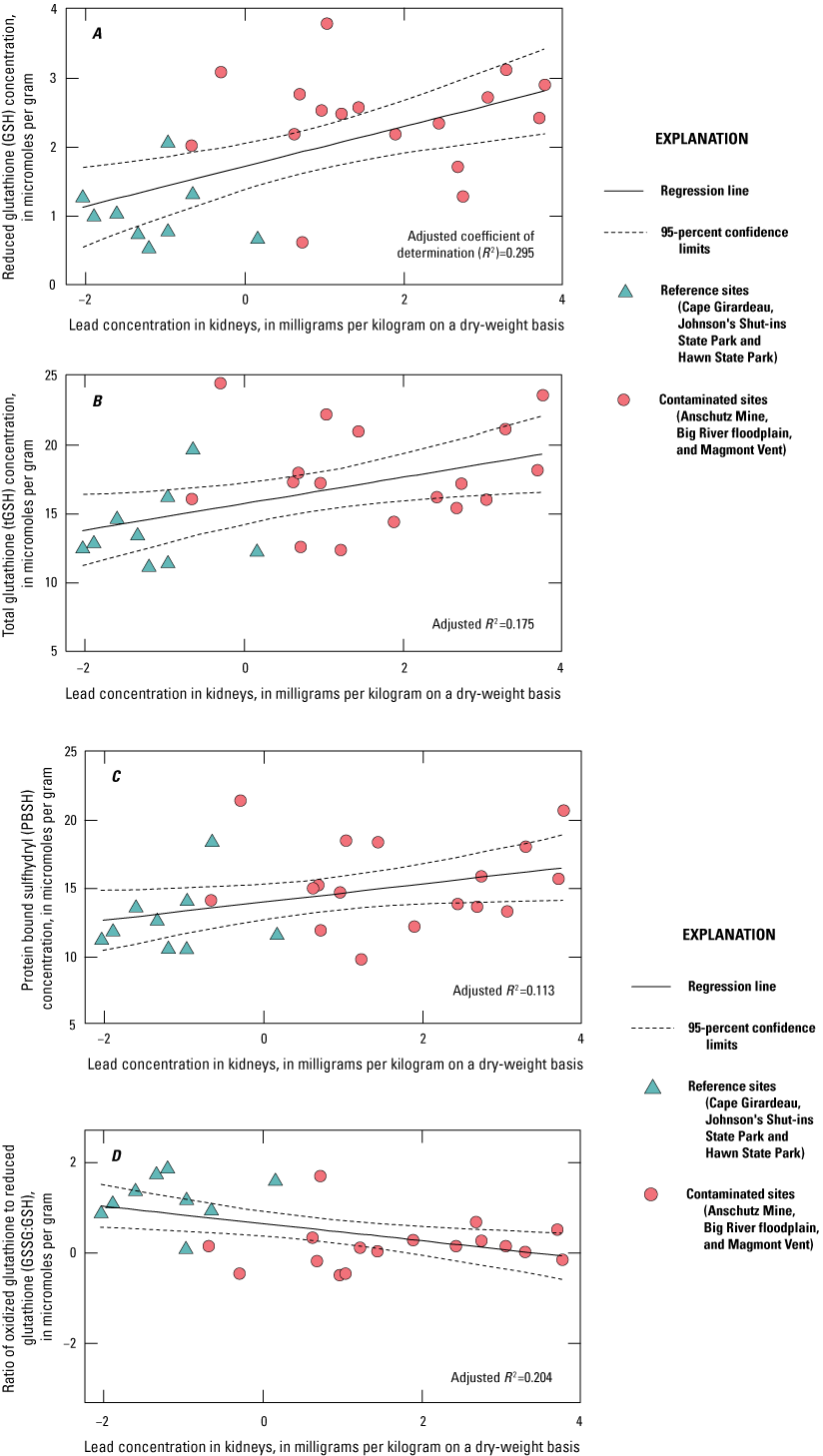
Relations between oxidative stress markers and lead concentrations in kidneys of Cardinalis cardinalis (Linnaeus, 1758) (northern cardinal) in the Southeast Missouri Lead Mining District. A, reduced glutathione (GSH). B, total sulfhydryl (TSH). C, protein-bound sulfhydryl (PBSH). D, ratio of oxidized glutathione to GSH (GSSG:GSH).
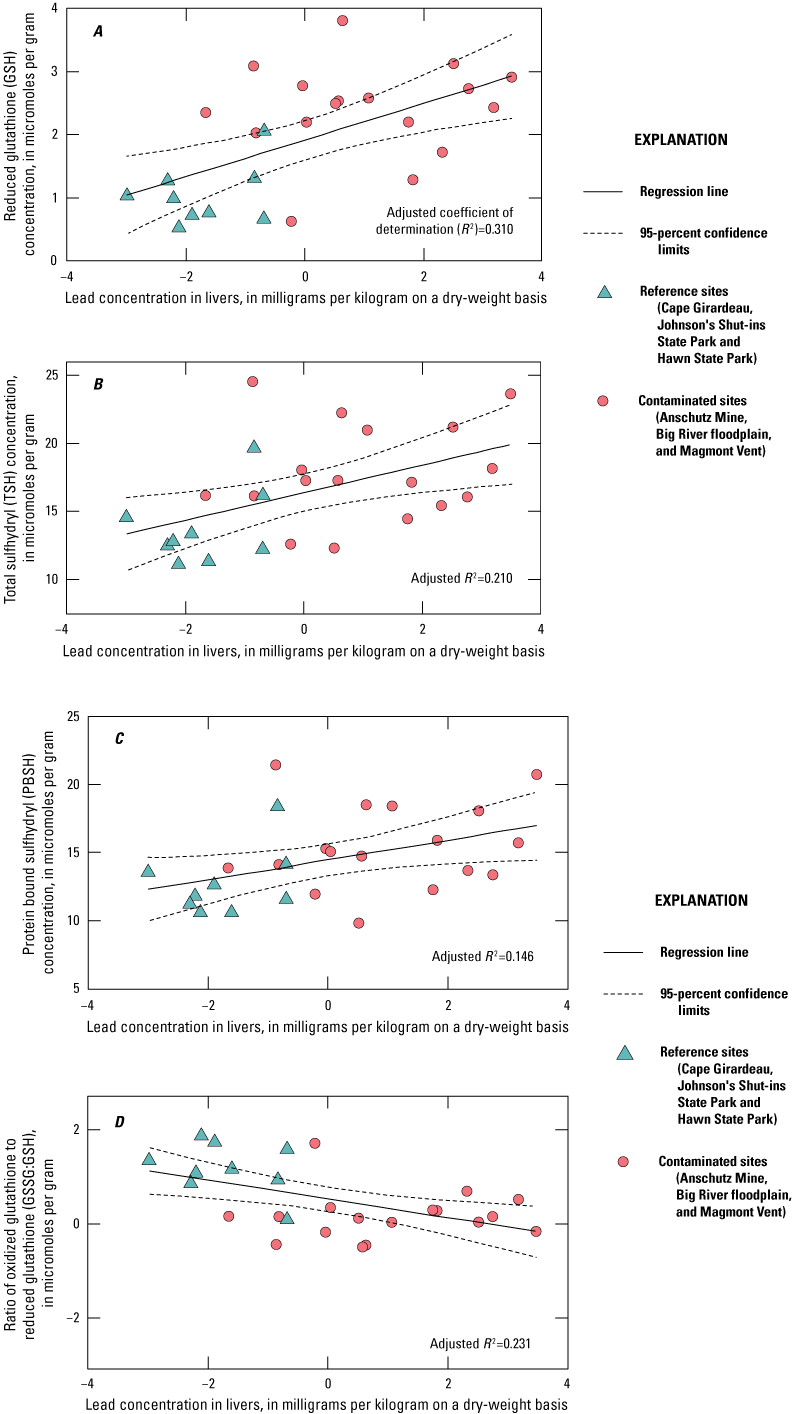
Relations between oxidative stress markers and lead concentrations in livers of Cardinalis cardinalis (Linnaeus, 1758) (northern cardinal) in the Southeast Missouri Lead Mining District. A, reduced glutathione (GSH). B, total sulfhydryl (TSH). C, protein-bound sulfhydryl (PBSH). D, ratio of oxidized glutathione to GSH (GSSG:GSH).
Microscopic Lesions in Liver and Kidney Tissues
The prevalence of microscopic changes within the livers and kidneys of birds from contaminated and reference sites was similar (table 21), and it was not possible to definitively link lesion occurrence with lead concentrations in blood or tissues in individual birds (individual bird data not shown; see Cleveland and others, 2023). Microscopic lesions in birds from contaminated and reference sites were often tied to parasitic infections, including inflammation and degeneration or necrosis in the kidneys and livers with intralesional protozoa in northern cardinals, and hepatic necrosis with nematode parasites in American robins. Other microscopic changes were consistent with common background lesions in wild birds. These included inflammation in the livers and kidneys of mourning doves and inflammation and necrosis in the livers of brown-headed cowbirds, particularly at Magmont Vent. The single brown-headed cowbird available from a reference site also had inflammation in its liver. We did not observe any acid-fast intranuclear inclusions in renal tubular epithelial cells in any bird.
Table 21.
Microscopic lesions in liver and kidney tissues of adult birds breeding in the Southeast Missouri Lead Mining District.[Data are summarized from Cleveland and others (2023). Notations indicate the number of individual birds having absent–present lesion occurrences (percentage [%] of total number of samples having the lesion present). NEC, degeneration/necrosis; INF, inflammation; HEMO, hemosiderosis; BS, bile stasis; LIP, lipidosis; BHY, biliary hyperplasia; MGY, cyto/karyomegaly; GLOM, glomerulopathy; AFI, acid fast inclusions]
Liver lesions are denoted as follows: NEC is hepatocellular degeneration or necrosis; INF is inflammation of any type and location, including granulomas, granulocytic inflammation, and Kupffer cell hyperplasia; HEMO is increased amounts of golden-green pigment in Kupffer cells or hepatocytes; BS is increased amounts of yellow-green pigment in bile canaliculi or hepatocytes; LIP is discrete clear vacuoles within hepatocytes; and BHY is increased numbers of bile duct profiles in portal triads.
Kidney lesions are denoted as follows: NEC is renal tubular degeneration or necrosis; INF is interstitial or tubular inflammation of any type, including lymphoplasmacytic inflammation, granulocytic inflammation, and granulomas; MGY is enlargement of cells or nuclei of renal tubular epithelium; GLOM is any glomerular abnormality; and AFI are discrete acid-fast positive intranuclear inclusions consistent with lead inclusions in the renal tubular epithelium.
Effects of Lead on Reproductive Success
The numbers of nests monitored for each species are reported in table 2; we monitored 95 eastern bluebird nests and 484 nests of the 4 open-cup species (eastern towhee, field sparrow, indigo bunting, northern cardinal). Twenty-two of 95 eastern bluebird nests failed (23 percent); 15 failures were due to abandonment, 5 were due to predation, and 2 failed due to weather-related events. In total, 320 open-cup nests failed (65 percent): 34 to abandonment, 283 to predation, and 3 to weather-related events. Also, 19 of the 484 open-cup nests were parasitized and had 1 brown-headed cowbird egg or nestling in them.
Descriptive statistics indicated that, based on successful nests, means for clutch size, proportion hatched, number of young fledged based on all nests, and number of young fledged were greater at reference sites than at contaminated sites in 17 of 20 comparisons (table 22). Nonetheless, we used more robust statistical inferences, based on GLM model results, to examine the effects of lead concentrations in soil and blood on reproductive success because the GLM models directly relate reproductive success to lead concentrations while controlling for site and year effects. Mean local lead concentrations in soil around nests ranged from 20 to 4,468 ppm for eastern bluebird; 19 to 4,165 ppm for eastern towhee; 19 to 4,295 ppm for field sparrow; and 19 to 4,280 ppm for indigo bunting. Mean local lead concentrations for northern cardinal nests ranged from 19 to 4,297 ppm, except for a single extreme value of 12,488 ppm. We removed this single extreme lead concentration from further analysis of bird reproductive success because it only occurred for one individual at a single point location, was extreme compared to all other values, and likely resulted from a unique, highly localized condition.
Table 22.
Sample sizes, means, and standard errors for estimates of reproductive success of songbird nests in reference and contaminated sites in the Southeast Missouri Lead Mining District.[Descriptive statistics for measures of reproductive success for focal species with greater than 30 nests monitored across reference sites and contaminated sites]
Clutch Size
Clutch size was determined using 24 to 95 nests per species; arithmetic means for clutch size ranged from 2.61 to 4.33 among species and were lower at contaminated sites compared to reference sites for all 5 species (table 22). We successfully fit models relating concentrations of lead in adult blood and local soil lead concentration to clutch size of eastern bluebirds and found no strong support for lead effects on clutch size (tables 23 and 24). The null models with no lead effect had lower DICs than models with BloodPb or SoilPb terms (change in [Δ] DIC=1.52 and 1.36, respectively), indicating a lack of support for a lead effect. The credible intervals for the effect of BloodPb and SoilPb overlapped zero, and posterior distributions indicated a low probability of negative effects of BloodPb (0.64 probability) and SoilPb (0.56 probability) (tables 23 and 24). Similarly, there was no strong support for an effect of local lead concentration on clutch size of any of the open-cup nesting species. The null model without SoilPb effects had a lower DIC than the model with SoilPb effects (ΔDIC=6.67), indicating a lack of support for a lead effect. Credible intervals for the mean effect of SoilPb on each species overlapped zero. Posterior distributions indicated a 0.56, 0.73, and 0.67 probability of a negative effect on field sparrows, eastern towhees, indigo buntings, respectively, and a 0.52 probability of a positive effect of SoilPb on northern cardinals (table 25).
Table 23.
Effect sizes from linear models relating lead concentrations in blood to reproductive measures of Sialia sialis (Linnaeus, 1758) (eastern bluebird) in the Southeast Missouri Lead Mining District.[Results of Bayesian analysis of generalized mixed linear models including year and site as random effects. Lead concentrations were logarithmically (base 10)-transformed prior to modeling. SD, standard deviation; LCR, lower credible interval; UCR, upper credible interval; Propn, proportion of the distribution of the estimate with the same sign as the mean; BloodPb, lead concentration in blood, in milligrams of lead per kilogram of blood on a dry-weight basis]
Intercept and adult BloodPb are the model intercept and fixed effect of adult blood lead concentration for each reproductive measure, respectively.
Table 24.
Effect sizes from linear models relating the lead concentration in soil to reproductive measures of Sialia sialis (Linnaeus, 1758) (eastern bluebird) in the Southeast Missouri Lead Mining District.[Results of Bayesian analysis of generalized mixed linear models including year and site as random effects. Lead concentrations were logarithmically (base 10)-transformed prior to modeling. SD, standard deviation; LCR, lower credible interval; UCR, upper credible interval; Propn, proportion of the distribution of the estimate with the same sign as the mean; SoilPb, lead concentration soil, in milligrams of lead per kilogram of soil on a dry-weight basis]
Intercept and SoilPb are the model intercept and fixed effect of soil lead concentration for each reproductive measure, respectively.
Table 25.
Effect sizes from linear models relating lead concentrations in soil to reproductive measures of four open-cup nesting songbird species in the Southeast Missouri Lead Mining District.[The four species are Spizella pusilla (A. Wilson, 1810) (field sparrow), Pipilo erythrophthalmus (Linnaeus, 1758) (eastern towhee), Passerina cyanea (Linnaeus, 1766) (indigo bunting), and Cardinalis cardinalis (Linnaeus, 1758) (northern cardinal). Results of Bayesian analysis of generalized mixed linear models including year and site as random effects. Lead concentrations were logarithmically (base 10)-transformed prior to modeling. SD, standard deviation; LCR, lower credible interval; UCR, upper credible interval; Propn, proportion of the distribution of the estimate with the same sign as the mean; SoilPb, lead concentration in soil, in parts per million; ×, denotes an interaction between the two variables]
Intercept, Species, SoilPb, and Species × SoilPb are fixed effects in the model for each reproductive measure. Because Species was a categorical variable representing four species, field sparrow was defined as the reference category. The interaction of the Species × SoilPb represents how the effect of SoilPb on that species differs from the reference species, field sparrow, and SoilPb is the effect of SoilPb on field sparrow. The parameter of primary interest is “SoilPb on Species name,” which represents the effect of SoilPb on the reproductive measure for that species and is calculated as the SoilPb effect plus the interaction effect for that species.
Hatching Success
The proportion of eggs hatched (hatching success) was determined by species (n=24 to 95 nests per species). Simple arithmetic means ranged from 0.82 to 0.94 and were similar for contaminated and reference sites (table 22). There was no strong support for effects of lead in blood or local soil on the hatching success of eastern bluebirds. The models with no lead effects had lower DICs than models that included BloodPb or SoilPb (ΔDIC=1.52 and 1.48, respectively), and the credible intervals for the mean effect of BloodPb and SoilPb overlapped zero. However, posterior distributions indicated a 0.75 and 0.78 probability of a negative effect of BloodPb and SoilPb, respectively, on hatching success (tables 23 and 24). Similarly, there was no strong support for an effect of local soil lead concentration on hatching success of any of the open-cup nesting species. The model with no SoilPb effects had a lower DIC than the model with SoilPb and SoilPb × Species (ΔDIC=8.06); the credible intervals for the mean effect of SoilPb on each species overlapped zero. Posterior distributions indicated a 0.67 and 0.54 probability of a negative effect on eastern towhees and indigo buntings, respectively, and 0.70 and 0.73 probability of a positive effect on field sparrows and northern cardinals, respectively (table 25).
Number of Young Fledged
The number of young fledged was determined for 47 to 176 nests per species when all nests were considered, and for 10 to 73 nests per species when only successful nests were considered. Mean numbers of young fledged per species ranged from 0.39 to 2.78 for all nests and from 2.40 to 3.68 for successful nests (table 22). The mean number of young fledged was lower on contaminated sites than reference sites for all 5 species when considering all nests, and for 4 of 5 species (all except eastern bluebird) when considering successful nests only; however, standard errors were large, indicating a considerable amount of variation (table 22).
There was no strong support for effects of lead in blood or local lead concentration in soil on number of young fledged for eastern bluebirds (tables 23 and 24). Models that included BloodPb or SoilPb had greater DIC values than the null models with no lead effects included (ΔDIC=1.64 and 1.59, respectively). The credible intervals for the mean effects of BloodPb and SoilPb overlapped zero, and posterior distributions indicated a 0.54 and 0.60 probability of a negative effect of BloodPb or SoilPb, respectively (tables 23 and 24). Similarly, there was no support for an effect of local soil lead concentration on number of young fledged for any of the open-cup nesting species. The model with no SoilPb effects had a lower DIC than the model with SoilPb and SoilPb × Species (ΔDIC=6.79). Credible intervals for the mean effect of SoilPb on each species overlapped zero. Posterior distributions indicated a 0.77, 0.66, and 0.54 probability of a negative effect on field sparrows, eastern towhees, and northern cardinals, respectively, and 0.55 probability of a positive effect on indigo buntings (table 25).
Nest Success
We monitored the success of 95 eastern bluebird nests over a total of 574 nest-check intervals. The relation between eastern bluebird nest success and lead concentration in blood could not be estimated because the model would not converge on a solution. This was likely because of a relatively small sample size (number of nests with BloodPb values=66) and a high nest success rate (only 22 of 95 nests failed). We successfully fit a model for the effect of SoilPb on nest success of eastern bluebirds, and there was no support for the effect of local lead concentration. The model with no SoilPb effect had a lower DIC than the model with SoilPb (ΔDIC=1.22). The credible interval for the mean effect of SoilPb overlapped zero, and the posterior distributions indicated a 0.56 probability of a negative effect of SoilPb (table 24). Mean daily nest survival of eastern bluebirds was 0.998, and the period nest survival was 0.928 (table 24).
We found support for an effect of local lead concentration in soil on nest survival of some open-cup nesting birds; in other words, the nest survival of some species decreased as the local lead concentration in soil increased. We monitored success of 484 open-cup nests over a total of 2,084 nest-check intervals; the number of nests ranged from 52 to 184 per species. Mean daily nest survival of open-cup nesting species was 0.913 to 0.934 (table 25), and the period nest survival was 0.095 to 0.202. The model with SoilPb effects had more support than a model without SoilPb effects (ΔDIC=2.04). The credible intervals for the mean effect of SoilPb on each species overlapped zero for all species except indigo bunting. However, posterior distributions indicated a 0.97, 0.99, and 0.67 probability of a negative effect of SoilPb on eastern towhees, indigo buntings, and northern cardinals, respectively (table 25). As a result of the predicted effect of SoilPb, period nest survival for eastern towhees and indigo buntings decreased from >20 to <5 percent as local lead concentration in soil increased from 18 to 4,400 ppm (fig. 17). Period nest survival for northern cardinals decreased from 23 to 14 percent as the local lead concentration in soil increased from 18 to 4,400 ppm (fig. 17). Conversely, nest survival of field sparrows increased from 20 to 27 percent as lead concentrations in soil increased from 18 to 4,400 ppm (fig. 17).
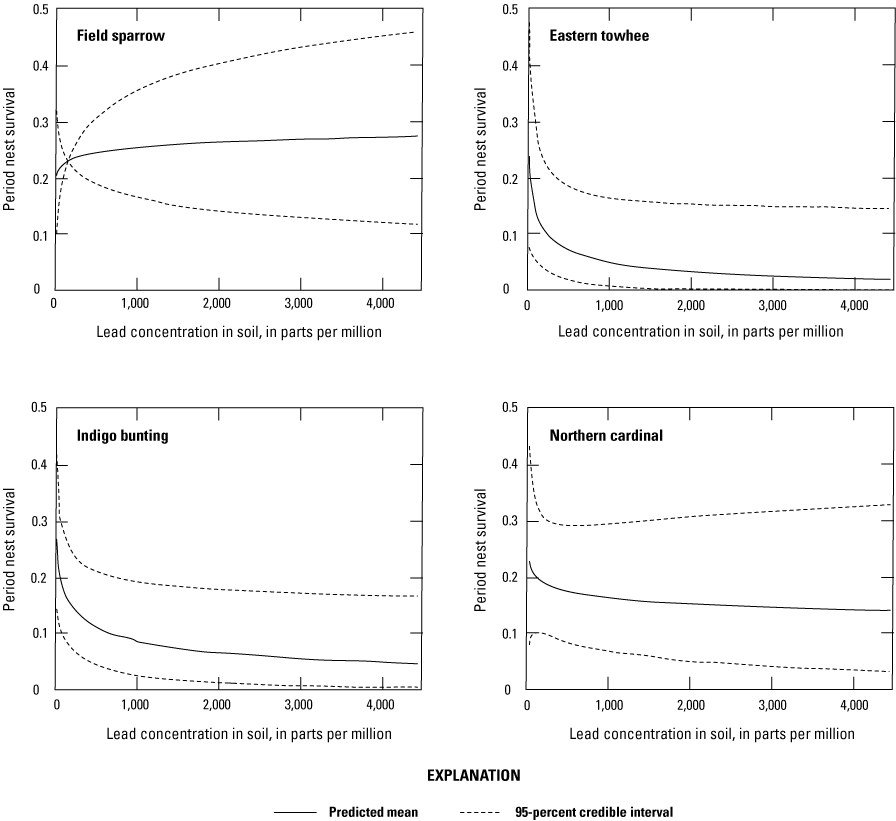
Relations between lead concentrations in soil and period nest survival of four open-cup nesting songbird species at the contaminated and reference sites in the Southeast Missouri Lead Mining District.
Habitat Effects on Reproductive Success
Nest survival was the only reproductive success measure for which there was statistically significant support for an effect of local lead concentration in soil; therefore, we considered whether any of the habitat variables explained additional variation in nest survival. However, the addition of nest concealment, percent ground cover, percent shrub cover, number of sapling-sized trees, number of pole timber-sized trees, or number of saw timber-sized trees to the nest survival model for open-cup nesting birds did not improve the model. The ΔDIC value for the addition of each variable ranged from 0.01 to 1.93, indicating that the model with a habitat effect added was no better or worse than the model without the habitat effect; moreover, addition of habitat effect did not help explain variation in nest survival. Furthermore, no habitat effects had credible intervals that did not overlap zero. Although habitat did not explain additional variation in nest survival, there were some patterns in habitat variables around nests based on examination of descriptive statistics (table 26). Percent ground cover was consistently lower for all species on contaminated sites (31 to 53 percent) than on reference sites (43 to 84 percent). In addition, numbers of sapling-sized trees were consistently lower for all species on reference sites (120 to 842) than on contaminated sites (423 to 1,187). The remaining habitat variables did not vary consistently among species between reference and contaminated sites, and the 95-percent confidence intervals for the means of all habitat variables overlapped between reference and contaminated sites (table 26).
Table 26.
Mean habitat characteristics at nests in the reference and contaminated sites in the Southeast Missouri Lead Mining District.[Habitat characteristics were evaluated according to the methods of Roach and others (2018) for four species: Pipilo erythrophthalmus (Linnaeus, 1758) (eastern towhee), Spizella pusilla (A. Wilson, 1810) (field sparrow), Passerina cyanea (Linnaeus, 1766) (indigo bunting), and Cardinalis cardinalis (Linnaeus, 1758) (northern cardinal). Mean, least-squared mean; SE, standard error; CI, 95-percent confidence interval range]
Discussion of Lead and its Effects on Birds Breeding in the Southeast Missouri Lead Mining District
Discussion of Lead and its Effects on Birds Breeding in the DistrictLead in Soil
Beyer and others (2013) concluded that lead concentrations ≥1,000 mg/kg dw (assumed to be equivalent to 1,000 ppm measured by pXRF) in soil in southeast Missouri were sufficient to expose ground-foraging birds to toxic lead concentrations, as evidenced by δALAD inhibition, elevated lead concentrations in tissues, and the presence of renal acid-fast inclusions in birds from contaminated sites compared to reference sites. None of our reference soils had lead concentrations that exceeded the 1,000 mg/kg dw threshold, whereas a total of 17 percent (n=66 of 394) of the soil samples at Anschutz Mine, 74 percent (n=258 of 347) at Magmont Vent, and 70 percent (n=258 of 370) at Big River floodplain had lead concentrations that exceeded the 1,000 ppm threshold by factors of as much as 35, 19, and 3, respectively (table 6).
However, the mean lead concentration in soil only exceeded 1,000 ppm at Big River floodplain and Magmont Vent (table 6). This discrepancy between mean concentrations, concentration ranges, and threshold exceedances further demonstrates the heterogeneity of lead in soils across the contaminated sites and supports the use of local lead concentrations in soil for assessment of reproductive effects. In other words, a site-wide mean soil lead concentration calculated for a heterogenous site may overestimate or underestimate effects depending on the exact location of a nest within a site, whereas raster grids estimate the lead concentrations in soil within the nesting territories, such that effects can be more closely linked to exposure.
We found support for some species effects in the relation of lead concentration in blood to lead concentration in soil and weak to moderate support for interactions between species and lead concentration in soil. Therefore, although lead concentration in blood differed among species for a given lead concentration in soil, the rate at which lead concentrations in blood increased in relation to lead concentrations in soil was similar across species and sites. Mean lead concentrations in blood ranged from <0.1 mg/kg dw in multiple species to 4.76 mg/kg dw in eastern bluebird broods (table 8). Differences in the lead concentrations in blood among species likely resulted from variability in interspecific foraging behaviors, diet, rate of soil ingestion, and local lead concentrations in soil (Beyer and others, 1994; Hansen and others, 2011). For example, at the Anschutz Mine site, American robins had a mean lead concentration in blood of 2.04 mg/kg dw, and northern cardinals had a mean concentration of lead in blood of 0.61 mg/kg dw (table 8). Both species are ground and arboreal foragers (table 1), but cardinals are generally opportunistic and will consume buds and insects on trees as the canopy develops (Halkin and Linville, 1999). In contrast, American robins feed heavily on earthworms during the breeding season (Beyer and Sample, 2017). In addition, high-protein diets may be protective against lead absorption (Franson and Pain, 2011); for example, Kendall and others (1982) observed that lead-dosed ringed turtle doves fed a mash diet with 27 percent protein had substantially lower lead concentrations in their tissues compared to birds fed corn (low protein). The authors hypothesized that protein-induced secretion of pepsin in the gastrointestinal tract and the thiol groups on the pepsin molecules chelated the lead as it dissolved.
Previous studies have collected and analyzed ingesta in euthanized adult birds to estimate the rate of incidental soil ingestion (and subsequently ingestion of lead in soil) by individuals (Grue and others, 1984, 198672; Blus and others, 1995). Hansen and others (2011) found that soil ingestion rate differs greatly among species; American robins had an estimated soil ingestion rate of 20 percent, whereas Swainson’s thrush had a soil ingestion rate of 0.7 percent. Both American robins and Swainson’s thrush forage on the ground; however, Swainson’s thrush gleans food items from litter and foliage, whereas the foraging strategy of robins includes probing their bills into the soil. Further, soil ingestion rates likely fluctuate daily and seasonally as individuals alter their diet in response to food availability. For example, the proportion of fruit in the diet of the American robin is much greater in the fall and winter compared to the spring and summer (Wheelwright, 1986). Precipitation events may also affect soil ingestion rates; earthworms emerge during rain, becoming more readily available to birds. Rainwater may wash the exterior of the worms, reducing the amount of soil ingested by the bird; in contrast, immediately after rains, the soils may more readily stick to the wet earthworm bodies, increasing the amount of soil ingested by the bird. However, we did not evaluate the overall dietary composition of our study birds, nor did we evaluate the amount of external or internal sediment on the invertebrate bodies.
Variability in lead concentrations in blood among species and individuals can also depend on the bioavailability of the lead, particularly because of lead speciation and solubility (Hemphill and others, 1991; Kastury and others, 2019). Factors that affect lead bioavailability in soils include the lead concentration, the organic carbon concentration in the soil, soil pH, clay content, cation exchange capacity, particle size, and other predominant cations and anions in the soil (Hettiarachchi and Pierzynski, 2004; Ming and others, 2012; Yan and others, 2017). We believe that spatial differences in lead bioavailability within and among sites likely affected the uptake of soil-borne lead in our study animals.
Relative absorption rates of dietary lead are also affected by the lead compound (for example, metallic lead versus lead salts); for example, kidney absorption of lead from lead carbonate is twelvefold that of lead from metallic lead and 1.6 times that of lead from lead acetate in rats (Barltrop and Meek, 1975). Lead carbonate and lead acetate are readily soluble in the digestive system and, therefore, generally have a high degree of bioavailability via gastrointestinal absorption (Casteel and others, 2006; Scheckel and others, 2009); indeed, lead acetate is considered to have 100 percent relative bioavailability (RBA). Lead speciation and bioavailability studies have been previously conducted on soils in mining districts in Missouri, including the Southeast Missouri Lead Mining District (Yang and others, 2002; Casteel and others, 2006; Weber and others, 2015; Beyer and others, 2016). Lead-contaminated soils in Missouri generally have elevated concentrations of lead carbonate because the soils are weathered from limestone or dolomite, or from limestone or dolomite forming the host rock for the ore; these elevated concentrations resulted in the surficial presence of weatherable, soluble lead-bearing minerals in the mine/mill wastes (Weber and others, 2015).
Beyer and others (2016) dosed Japanese quail with soil from several lead-contaminated sites from around the United States, including from the Big River floodplain (Washington State Park, Desoto, Mo.) and Magmont Vent sites. Lead concentrations in blood of quail that had been dosed with lead acetate (100 percent RBA), were compared with lead concentrations in blood of quail dosed with field-contaminated soils. The mean lead concentration in blood samples from quail fed lead acetate (41 mg/kg dw lead in feed; 100 percent RBA) was 1.25 mg/kg dw. When soil from the Big River floodplain and Magmont Vent study sites were added to control diets, the mean lead concentrations in blood in quail increased from 0.019 mg/kg dw (with the control diet) to 1.65 mg/kg dw (86 mg/kg dw lead in feed) and to 2.26 mg/kg dw (137 mg/kg dw lead in feed), respectively (Beyer and others, 2016). The lead in soil at Big River floodplain had 63 percent RBA, and the Magmont Vent soil had 54 percent RBA; soils from Anschutz Mine were not evaluated as part of that study. The authors noted differences in minerology and co-occurring contaminants between the Big River floodplain and Magmont Vent soils, which may have affected the RBAs (Beyer and others, 2016). The mean lead concentrations in the blood of birds at the Big River floodplain and Magmont Vent sites measured in the present study (0.27 to 6.34 mg/kg dw; table 8) were generally consistent with those quantified by Beyer and others (2016), which supports the hypothesis that our target birds were exposed to bioavailable lead in soils in the Southeast Missouri Lead Mining District. Although we did not evaluate the mineralogy of our soil samples, differences among study sites likely affected our results in a similar manner to Beyer and others (2016). A discussion of the other potentially toxic metals (cadmium, zinc, copper, nickel, and cobalt) in soil, invertebrates, and blood at the study sites and their potential effects on the toxicity and bioavailability of lead is presented in appendix 3.
We used a simplified bioavailability metric that calculated the ratios of mean lead concentrations of blood to mean lead concentrations in soil (BloodPb:SoilPb, termed “bioavailability ratio”) for species common to all sites (eastern bluebird, indigo bunting, and northern cardinal) to compare relative uptake of lead. Birds exposed to Big River floodplain soils had the greatest mean bioavailability ratios (0.001 to 0.004). The bioavailability ratios at Anschutz Mine and Magmont Vent were less than or equal to 0.001. These results suggest that lead in Big River floodplain soils had greater bioavailability to birds than soils from the other sites, and this finding is consistent with the laboratory studies performed by Beyer and others (2016).
The EPA performed a site-specific baseline ecological risk assessment (BERA) for the Big River Mine Tailings (Superfund) Site (EPA, 2006; https://cumulis.epa.gov/supercpad/cursites/csitinfo.cfm?id=0701639) and an ecological risk assessment (ERA) for Southwest Jefferson County Mining (Superfund) Site (EPA, 2020; https://cumulis.epa.gov/supercpad/cursites/csitinfo.cfm?id=0705443) to evaluate potential sources of contamination to the Big River watershed and floodplain. Both sites are in Missouri’s Old Lead Belt. Ecological risk assessments use site-specific media (in other words, earthworms, soil, water, vegetation, and so on) and literature-based ingestion rates for specific receptors to determine how the average daily dose of lead (or other target metals) at the site compares to literature-based values for the no-observed-adverse-effect level and the lowest-observed-adverse-effect level (LOAEL) toxicity reference values (TRVs) (EPA, 1997).
For the Big River Mine Tailings Site BERA (EPA, 2006), a LOAEL TRV dose of 11.3 milligrams of lead per kilogram of body weight per day (mg/kg BW/d) was used, which Edens and others (1976) found resulted in a decrease in egg hatching success in Japanese quail. The BERA also found that site-specific lead concentrations in soil (353 mg/kg dw) and depurated earthworms (126 mg/kg dw) for the Big River Mine Tailings Site (St. Francois County, Mo.) exceeded the LOAEL TRV for Scolopax minor (J. F. Gmelin, 1789) (American woodcock) by a factor of nine. In other words, the site presents a risk to avian vermivores because of ingestion of lead in earthworms and soil when lead concentrations in soil and earthworms exceed 353 mg/kg dw and 126 mg/kg dw, respectively. We found that >70 percent of soil samples (n=787 of 1,110) collected from our contaminated study sites exceeded this 353 mg/kg lead threshold; 100 percent (n=15 of 15) of invertebrates from the contaminated sites exceeded the 126 mg/kg dw threshold. Therefore, the birds at our contaminated study sites were likely adversely affected by soil- and invertebrate-borne lead, assuming soil ingestion rates similar to the defaults used in the ERA models.
The Southwest Jefferson County Mining (Superfund) Site ERA (EPA, 2020) differed from the Big River Mine Tailings Site BERA (EPA, 2006) in that for the former, the EPA (1) used nondepurated, unwashed worms to determine the ADD, (2) included American robins in their avian risk analysis, and (3) used a greater LOAEL TRV (27.4 mg/kg BW/d lead) than was used for the Big River Mine Tailings Site BERA (11.3 mg/kg BW/d lead). The increased LOAEL TRV value (27.4 mg/kg BW/d) was based on egg production in chickens (Sample and others, 2019). The earthworm concentration used for the average daily dose in the Southwest Jefferson County Mining (Superfund) Site ERA was 499.2 mg/kg dw lead. The resulting modeled dose to the American robin (using 499.2 mg/kg dw lead in undepurated earthworms) exceeded their adverse reproductive effects threshold for lead (27.4 mg/kg BW/d) by a factor of four. We found that >70 percent of the invertebrate samples collected from our contaminated study sites (n=12 of 17) exceeded the worm concentration (499.2 mg/kg dw) used in the Southwest Jefferson County Mining (Superfund) Site ERA, whereas no invertebrates from the reference site invertebrates exceeded the threshold (table 7).
Lead in Bird Tissues
Few published studies have reported lead concentrations in blood of songbird species. Indeed, this is the first known analysis of lead in brood blood for eastern towhees, field sparrows, indigo buntings, and northern cardinals. Broods generally had greater mean lead concentrations in blood at the contaminated sites; however, these values were not statistically tested because of small sample sizes for broods (table 8). Broods in 8 of 12 comparisons at the contaminated sites had greater mean lead concentrations in blood than their parents; however, the concentration ranges were similar between the age classes (table 8).
Adults have a restricted foraging range while tending to young in the nest. In this way, uptake of lead during the breeding season, as reflected by increased lead concentrations in blood, are likely caused by local exposure to lead contamination, rather than offsite exposures. Elevated lead concentrations in broods at contaminated study sites compared to the reference sites further demonstrate that exposure to and uptake of lead occurred locally, within the breeding territory. In other words, it is expected that broods would primarily be exposed to lead via ingestion of lead-contaminated food items collected by the adults within the breeding territory and around the nest location. Therefore, adults and their broods would be expected to have similar levels of exposure to local lead, resulting in similar lead concentrations in the blood.
Seasonal variations in diet and subsequent incidental soil ingestion may also result in differing exposures to lead, and temporal changes in lead concentrations in blood, among individual adults and their broods. For example, a study in Michigan found that earthworms constituted 10 percent of bluebird nestling diet in May but were completely absent from the diet in July (Pinkowski, 1978). Additionally, Pinkowski (1978) found that nestlings most-commonly consumed ground-dwelling spiders in the spring and plant-dwelling spiders in the summer. As previously mentioned, the diet of nestlings is restricted spatially and temporally; in this way, broods hatching earlier in the breeding season, when adults might provide nestlings with more ground-dwelling invertebrates, may have had more exposure to soil-borne lead that, consequently, caused greater lead concentrations in brood blood. However, we did not evaluate lead concentrations in blood temporally according to hatching date.
Currently (2022), no literature-based thresholds exist for harm to Passeriformes (songbirds) based on lead concentrations in their blood. Therefore, in accordance with previous songbird studies (Hansen and others, 2011; Sample and others, 2011), we used Franson and Pain’s (2011) suggested interpretations of lead concentrations in tissue (blood, livers, and kidneys) for the orders Anseriformes (waterfowl), Falconiformes (raptors), and Columbiformes (pigeons and doves) (table 27) to assess the potential for adverse effects. Note that the exact concentrations in each of the threshold ranges (no evidence of lead poisoning, subclinical poisoning, clinical poisoning, or severe clinical poisoning) depends on the avian order (Anseriformes, Falconiformes, or Columbiformes); the most sensitive order is Falconiformes. The clinical poisoning category represents the lead concentration in a tissue at which clinical manifestations of lead poisoning (for example, anemia, weight loss, and muscle incoordination) would be expected in adult birds. However, avian development stage can affect the extent of harm. For example, nestlings and fledglings are hypothesized to be more sensitive to lead exposure than adults, so adverse effects after exposure to lead may be more profound in broods compared to adults. Therefore, interpreting brood blood concentrations according to Franson and Pain (2011) may not be entirely suitable and may underestimate adverse effects in broods (and indeed not only for songbird broods but for Anseriformes, Falconiformes, and Columbiformes broods as well), but we hypothesize that it is a useful way to examine and compare nestling and adult exposure and is deserving of further investigation. In addition, we note that diet, size, metabolism, behavior, and habitat differ between songbirds and Anseriformes, Falconiformes, and Columbiformes; these differences, particularly increased exposures to soil-borne lead, may result in underestimation of risk to songbirds relative to other orders when using the interpretative ranges. Wild birds may also be more susceptible to lead toxicoses than the test birds and controlled exposure scenarios used to create these thresholds because of inadequate diets, stressors, and other factors (Franson and Pain, 2011).
Table 27.
Comparisons of literature-based toxicity thresholds with tissue lead concentrations in adult birds and their broods, by site type, in the Southeast Missouri Lead Mining District.[Data are summarized from Cleveland and others (2023). Literature-based interpretation range designations (“No evidence of lead poisoning,” “Sub-clinical poisoning,” “Clinical poisoning,” and “Severe clinical poisoning”) are based on the measured lead concentrations in tissues (table 8 for blood; table 9 for livers and kidneys) that could be interpreted as evidence of lead poisoning in orders Anseriformes, Falconiformes, and Columbiformes according to Franson and Pain (2011). Note that thresholds were provided on a wet-weight tissue basis and converted to a dry-weight basis using the following moistures for each tissue type: blood, 78.26 percent moisture; liver, 67.74 percent moisture; kidney, 76.74 percent moisture; Franson and Pain, 2011). There are no tissue-based thresholds specific to Passeriformes in the literature. mg/kg dw, milligram of lead per kilogram of tissue on a dry-weight basis; n, total number of samples analyzed for lead concentration; <, less than]
Blood concentrations <0.9 mg/kg dw lead are generally considered to be background (assuming 78.26 percent moisture; table 27). About 97 percent (n=102 of 105) of adult blood samples and 98 percent (n=48 of 49) of brood samples at the reference sites had lead concentrations consistent with background (table 27). The exceptions were 2 indigo bunting adults (1.80 and 2.06 mg/kg dw) at Johnson’s Shut-ins State Park, 1 American robin adult (0.91 mg/kg dw) at Cape Girardeau, and 1 eastern bluebird brood (1.25 mg/kg dw) at Hawn State Park. At the contaminated sites, lead concentrations in blood in 39.7 percent (n=85 of 214) of adult birds and 45.8 percent (n=22 of 48) of broods were consistent with background; 60.3 percent (n=129 of 214) of adult birds and 54.2 percent (n=26 of 48) of broods had lead concentrations in blood that were consistent with some level of lead poisoning (that is, subclinical, clinical, or severe clinical). Eight adults and two brood blood samples at the contaminated sites (table 27) had lead concentrations consistent with severe clinical poisoning.
Lead concentrations in 40.3 percent (n=27 of 67) of livers of adult birds at the contaminated sites were consistent with some degree of lead poisoning (subclinical, clinical, or severe clinical; table 27); 1 individual had a lead concentration in liver associated with severe clinical effects. A total of 59.7 percent (n=40 of 67) of adults had lead concentrations in liver consistent with background (<6.2 mg/kg dw, assuming 67.74 percent moisture); in contrast, 100 percent of all reference livers had lead concentrations consistent with background.
Lead concentrations in 25.4 percent (n=17 of 67) of adult kidneys from the contaminated sites were consistent with background concentrations (<6.2 mg/kg dw; table 27), whereas 95 percent (n=19 of 20) of reference kidneys had background lead concentrations. About 75 percent (n=50 of 67) of kidneys from contaminated sites had lead concentrations associated with adverse effects, and as much as 38.8 percent of adult kidneys had lead concentrations associated with severe clinical poisoning. We note that although we did not observe signs of clinical or severe clinical lead poisoning (for example, wing droop, lethargy, or emaciation) in our study birds, the biological significance of tissue-based lead concentrations in birds may be difficult to ascertain for an individual bird with unknown history and chronicity of lead exposure (Franson and Pain, 2011). Many factors affect the absorption of lead in tissues. However, the results for lead in blood, paired with results for δALAD activity and other markers of biochemical damage (described in subsequent sections), suggests injury to birds at our contaminated study sites.
We also examined the interpretative benchmarks by species and site (table 28). Using the blood benchmarks, we found that several adults or broods were categorized as having been severely clinically poisoned; these individuals were sampled at Anschutz Mine (1 adult American robin and 1 adult mourning dove), Big River floodplain (1 adult eastern bluebird, 2 eastern bluebird broods, and 4 adult northern cardinals), and Magmont Vent (1 adult mourning dove). Adult birds having lead concentrations in blood suggestive of clinical lead poisoning were found at all three contaminated sites (table 28). Further, each contaminated site had at least one brood having a lead concentration in blood suggestive of some degree of poisoning (that is, subclinical, clinical, or severe clinical). Clinically poisoned broods were found at Anschutz Mine (1 eastern bluebird brood and 2 field sparrow broods), Big River floodplain (1 indigo bunting brood), and Magmont Vent (1 eastern bluebird brood and 1 eastern towhee brood); subclinically poisoned broods were found at Anschutz Mine (5 eastern bluebird broods, 1 eastern towhee brood, 2 field sparrow broods, and 1 northern cardinal brood), Big River floodplain (1 indigo bunting brood), and Magmont Vent (4 eastern bluebird broods, 1 field sparrow brood, and 2 northern cardinal broods).
Table 28.
Species-based interpretations of concentrations of lead in tissues of adult birds and their broods in the Southeast Missouri Lead Mining District.[Data are summarized from Cleveland and others (2023). %, percentage of samples having a lead concentration in the specified interpretation range; —, no data]
Literature-based interpretation range designations (“No evidence of lead poisoning,” “Sub-clinical poisoning,” “Clinical poisoning,” and “Severe clinical poisoning”) are based on the measured concentrations of lead in tissues (table 8 for blood; table 9 for livers and kidneys) that could be interpreted as evidence of lead poisoning in the order Falconiformes according to Franson and Pain (2011). Note that thresholds were provided on a wet-weight tissue basis and converted to a dry-weight (dw) basis using the following moistures for each tissue type: blood, 78.26 percent moisture; liver, 67.74 percent moisture; kidney, 76.74 percent moisture; Franson and Pain, 2011). No evidence of poisoning corresponds with concentrations of lead of <0.9 milligram per kilogram (mg/kg) dw in blood, and <6.2 mg/kg dw in livers and kidneys. Subclinical poisoning corresponds with lead concentrations of 0.9–2.3 mg/kg dw in blood, 6.2–18.6 mg/kg dw in livers, and 6.2–12.4 mg/kg dw in kidneys. Clinical poisoning corresponds with a lead concentration 2.3–4.6 mg/kg dw in blood, 18.6–31.0 mg/kg dw in livers, and 12.4–18.6 mg/kg dw in kidneys. Severe clinical poisoning corresponds with a lead concentration >4.6 mg/kg dw in blood, >31.0 mg/kg dw in livers, and <18.6 mg/kg dw in kidneys.
We found that the use of the liver and kidney poisoning benchmarks (Franson and Pain, 2011) changed not only the numbers of individuals considered to have been adversely affected compared to the blood benchmarks (table 28), but also the number of exceedances varied by choice of liver or kidney benchmark. For example, 1 American robin adult had lead concentration in its blood that was suggestive of severe clinical poisoning, no American robin livers had lead concentrations indicative of severe poisoning, and 12 American robin kidneys had lead concentrations suggestive of severe poisoning. It is likely that lead in the blood was rapidly distributed to various other storage sites (for example, liver, bone, and kidneys; Eisler, 1988; De Francisco and others, 2003; Berglund and others, 2011). In addition, lead ingestion and uptake rates likely varied daily. Nevertheless, the measured lead concentrations in the tissues (blood, livers, and kidneys) of our study birds indicate that adults and their broods are exposed to and ingest lead at the contaminated sites, and these lead concentrations are potentially harmful.
δALAD Activity
The first measurable effects of lead absorption into the avian bloodstream is inhibition of several enzymes involved in heme synthesis, including δALAD, and enzyme inhibition can occur at <0.2 mg/kg dw lead in blood (assuming 78.26 percent moisture; Franson and Pain, 2011). δALAD activity is also inhibited in other tissues, including the liver, kidneys, and brain; moreover, the assay is not affected by age, sex, or breeding condition of birds (Finley and Dieter, 1978), which is why δALAD activity inhibition is often used as a sensitive biomarker of acute and chronic lead exposures. In total, 18 of 34 samples (53 percent) for all species combined at contaminated sites, and 8 of 16 samples (50 percent) for northern cardinals at contaminated sites, had δALAD activity <50 percent of that observed at the reference sites (table 18). Dieter and Finley (1979) observed a substantial increase of butyrylcholinesterase, a marker suggestive of brain damage, at 75 percent δALAD activity inhibition in blood; 13 of 34 study birds (38 percent) at the contaminated sites had >75 percent δALAD inhibition relative to the mean δALAD activity at the reference sites.
These findings also indicate the characteristic inverse relation between δALAD activity and lead concentrations in tissues (blood, livers, and kidneys) of the study species (fig. 12; tables 18 and 19); this is a well-known relation described in many studies (for example, Franson and Pain, 2011) that results from displacement of zinc, and replacement by lead, on the sulfhydryl group at the active site of the δALAD enzyme. Inhibition of δALAD activity can persist for weeks to months after exposure (Franson and Pain, 2011); further, although birds can tolerate some reductions in blood and tissue δALAD activity without effects on hematocrit and hemoglobin concentration or the presence of overt signs of lead intoxication, anemia can result from chronic δALAD inhibition. Lead inhibition of δALAD activity also results in the buildup of excess aminolevulinic acid substrate, which is associated with oxidative damage and cell death (Scinicariello and others, 2007). The results for δALAD, coupled with those for lead concentrations in the blood, liver, and kidney tissues of the study birds indicate that birds nesting at the contaminated sites are likely to experience adverse outcomes.
Oxidative Stress Indicators and DNA Damage
Environmental contaminants, like lead, can overwhelm the protective antioxidant systems that protect birds against normal oxidative processes (for example, those required for metabolism, immune defense), leading to a buildup of reactive oxygen species (ROS) that damage biomolecules (for example, cell injury or death, and DNA damage) and can affect immune function, growth, survival, and reproductive success (Koivula and Eeva, 2010). We observed similar patterns of expression of oxidative markers relative to reference samples between northern cardinals and American robins (table 20), although concentrations of the oxidative markers differed between the 2 species. Despite statistical differences only being clear for northern cardinals, concentrations of GSH, TSH, and PBSH in both species were greater in livers from contaminated sites compared to those from the reference sites. Lead binds the sulfhydryl groups of GSH and other thiols, depleting their stores in the liver and thereby interfering with their antioxidant activities (Ahamed and Siddiqui, 2007). Therefore, the increases that we observed may be indicative of compensatory measures taken to counter increased levels of ROS. Increased synthesis of GSH and other thiols may allow these birds to survive despite chronic exposure to elevated lead concentrations. Monaghan and others (2009) reported that prolonged elevation of ROS can cause a permanent increase in baseline antioxidant levels. This is supported by the positive relations that we observed between these indicators of thiol status (GSH, tGSH, TSH, PBSH) and lead concentrations in liver and kidney tissues. Furthermore, the GSSG:GSH ratio was lower in northern cardinals from contaminated locations compared to those from reference sites, largely because of greater GSH concentrations.
Basal (reference site) levels of GSH, tGSH, and TBARS were greater in American robins than in northern cardinals, whereas GSSG levels were lower. Differences in oxidative stress-related endpoints between bird species have been reported previously. For example, Guan (2010) found that GSH and TBARS differed among four avian species and postulated that longevity of the species may play a role in these differences. Isaksson and others (2017) reported differences in lipid peroxidation between four species of songbirds and attributed these in part to dietary variations that affect intake of antioxidants. Similarly, Rainio and others (2013) reported differences among songbird species for some indicators of oxidative stress but not for tGSH or the ratio of GSH:GSSG. This suggests that species may use varying strategies to regulate their oxidative state. Intra- and inter-specific differences in susceptibility to lead have also been noted in birds (Mateo and Hoffman, 2001).
Previous studies have shown substantially greater levels of DNA damage and lipid peroxidation in animals exposed to lead compared to reference animals (Mateo and others, 2003; Matović and others, 2015; Vallverdú-Coll and others, 2016). The DNA lesion 8-OH-dG is formed by the oxidation of the C-8 position of 2′-deoxyguanosine and is induced in the liver after exposure to lead. Formation of the 8-OH-dG lesion has been correlated with elevated levels of ROS in lead-treated rats (Liu and others, 2012). Lipid peroxidation is the process by which ROS attack unsaturated lipids producing lipid radicals (Ayala and others, 2014). Both DNA damage and lipid peroxidation occur under baseline conditions but can be repaired and maintained at low levels until repair capacity or compensatory mechanisms are overwhelmed. Neither lipid peroxidation nor DNA damage were increased in the lead-exposed birds of the present study, suggesting that the increased concentrations of GSH (and possibly other antioxidant species) were able to mitigate any oxidative stress generated by exposure to lead and reduced the amount of detectable tissue damage or reproductive impairment.
Microscopic Lesions in Liver and Kidney Tissues
Our soil and tissue results suggest that the lack of significant microscopic lesions that are generally associated with lead exposure (for example, acid-fast intranuclear inclusions or hepatocellular degeneration lacking an apparent etiology such as parasites) does not rule out the possibility of lead poisoning of birds in the Southeast Missouri Lead Mining District. We did not observe acid-fast intranuclear inclusions, a lesion previously associated with lead poisoning in mourning doves and other species (Kendall and others, 1983), in any of our study birds, despite the elevated lead concentrations in the tissues of the study birds at the contaminated sites (tables 8, 9). However, Beyer and others (2004) were similarly unable to link lesions to lead exposure in the Tri-State Mining District; and in the Southeast Missouri Lead Mining District, acid-fast inclusions were only observed in the two American robins with the greatest concentrations of renal lead (952 and 1,030 mg/kg dw; n=6 American robins of 34 total birds; Beyer and others, 2013). In the current study, the greatest renal lead concentration was 134 mg/kg dw in one mourning dove (table 9; Magmont Vent); the relatively low concentrations of renal lead measured in the current study compared to those measured by Beyer and others (2013) may explain the lack of intranuclear inclusions. It has also been documented that lesions may be less severe in birds with chronic lead poisoning compared to acute lead poisoning (Bono and Braca, 1973).
Moreover, little is known about the microscopic changes associated with lead poisoning in the species assessed in this study; lesion susceptibility may be species or tissue specific. For example, microscopic lesions were uncommon in the kidneys of lead-poisoned eagles; renal tubular necrosis is reported in only 5.4 percent of lead-poisoned Haliaeetus leucocephalus (Linnaeus, 1766) (bald eagles) and 17.6 percent of Aquila chrysaetos (Linnaeus, 1758) (golden eagles; Franson and Russell, 2014). In that study, microscopic lesions from lead poisoning were most common in the heart, a tissue that was not examined as part of this study.
Although inflammation and other lesions were common in the livers and kidneys of all four species examined (table 21), the prevalence of these changes was similar for birds at contaminated and reference sites. Further, the occurrence of lesions in individual birds did not seem to correspond to elevated lead concentrations in tissues; this suggests that the lesions are not likely caused by lead exposure. In northern cardinals and American robins, protozoan or metazoan parasites were present in many areas of inflammation and necrosis; these parasites were the most likely cause of these lesions. Some evidence indicates that birds exposed to lead experience immunosuppression and are more susceptible to parasitic infections (Vallverdú-Coll and others, 2019); moreover, birds experiencing oxidative stress may mount less effective immune responses to parasitic infection (Cram and others, 2015).
Effects of Lead on Reproductive Success
Simple comparisons of descriptive statistics indicated that species-specific means for clutch size, proportion hatched, number of young fledged based on all nests, and number of young fledged by successful nests of the five focal species (eastern bluebird, eastern towhee, field sparrow, indigo bunting, northern cardinal; table 22) were greater for reference sites than contaminated sites in 17 of 20 possible comparisons. However, when reproductive success was directly related to local lead concentration in soil while controlling for site and year in a GLM model, we found mixed support for effects on reproductive success. We found the strongest support for a negative effect of local lead concentration in soil on nest survival of open-cup nesting species. As previously described, our models indicated that period nest survival of eastern towhees and indigo buntings decreased from >20 to <5 percent each as local lead concentration in soil increased from 18 to 4,400 ppm. Period nest survival for northern cardinals was decreased from 19 to 13 percent based on a model with more support for SoilPb than a model without lead effects. However, these estimates had some uncertainty. Additionally, while there was a probability of a negative effect of SoilPb on the period nest survival for eastern towhees (0.97 probability), indigo buntings (0.99 probability), and northern cardinals (0.67 probability), the 95-percent credible interval for the mean effect overlapped zero for eastern towhees and northern cardinals. For field sparrows, there was a 70-percent probability of a positive relation between period nest survival and SoilPb concentrations. Nest success of eastern towhees, indigo buntings, northern cardinals, and field sparrows generally range from 18 to 45 percent in woodlands and field edges in other areas of Missouri with no known lead contamination (Woodward and others, 2001; Burhans and others, 2002; Peak and others, 2004; Roach and others, 2018). Nest success of the four open-cup nesters in this study was within the 18 to 45 percent range at low lead concentration in soil (<100 ppm), but was reduced to 2.0, 4.8, and 14.3 percent for eastern towhees, indigo buntings, and northern cardinals, respectively, when local lead concentrations in soil were ≥4,400 ppm.
The results for other reproductive measures were more equivocal. Overall, the models without lead effects for clutch size, hatching success, and number fledged had more support than models with lead effects. However, the differences in support were small (ΔDIC=1.2–1.6) for eastern bluebird, suggesting that models with lead effects should receive some consideration. Probabilities of a negative effect of local lead concentrations in soil on eastern bluebird reproductive measures were as great as 0.64 on clutch size and 0.78 for hatching success.
Pattee (1984) also did not find a difference in clutch size, clutch initiation date, or egg fertility between lead-dosed (50 ppm metallic lead) and control American kestrels. Similarly, in European starlings, there were no differences in clutch size, hatching success, or number and weight of 18-day-old nestlings between lead-contaminated (56 to 410 mg/kg dw lead in soil; 170 to 1,200 mg/kg dw lead in invertebrates) and control (16 to 34 mg/kg dw lead in soil; 1.7 to 58 mg/kg dw lead in invertebrates) sites (Grue and others, 1986). However, the mean brain weight of starling nestlings from the contaminated site (1.1 g) was significantly lower than the mean brain weight for control nestlings (1.3 g). Decreased brain weight associated with lead exposure has also been documented in the altricial nestlings of American kestrels and pied flycatchers (Hoffman and others, 1985a; Nyholm, 1998). This brain weight decrease suggests that brain development could be a more sensitive indicator of lead exposure than the reproductive parameters measured in this study.
Differences in the nesting ecology between the cavity-nesting eastern bluebird and the four open-cup nesting species (eastern towhee, field sparrow, indigo bunting, northern cardinals) likely affected the observed differences in relations of reproductive success with local lead concentrations. Nest predation is the primary cause of nest failure for songbirds (Ricklefs, 1969; Thompson, 2007; Remeš and others, 2012) and was the cause of most nest failures in this study. Indeed, nest survival was, on average, much lower for open-cup nesters (10 to 20 percent) than for eastern bluebirds (93 percent) even at reference sites. Nest box nests are considerably more protected from predators than open-cup nests, and the predator guards on our nest boxes provided nests with additional protection from predation. Open-cup nesting species were exposed to a similar range of local soil lead concentrations as eastern bluebirds. However, even at the minimum local lead concentration in soil (18 ppm), nest survival success of open-cup nesting species (17 to 23 percent) was much lower than the average nest survival of eastern bluebirds (93 percent), despite normalization with baseline predation levels, which supports the hypothesis that open-cup nesters were subjected to greater overall nest predation.
We believe that the observed disparity between effects on eastern bluebirds and open-cup nesting birds, and the observation that nearly all nest failures in open-cup nesting birds was from predation, is important. We hypothesize that lead exposure may affect adult and nestling behavior in such a way that makes nests more readily detectable by predators or that results in poorer nest defense behaviors. Lead affects the avian nervous system and can cause behavioral changes, including decreased food acquisition and poor coordination (Haig and others, 2014). Although birds have evolved approaches to reduce activity at the nest, which include altering incubation and feeding behavior and synchronization of parental behaviors (Conway and Martin, 2000; Leniowski and Węgrzyn, 2018), greater nest predation could result if lead poisoning impaired the ability of adult birds to perform or coordinate these behaviors. For example, increased parental activity at the nest can result in higher nest predation (Martin and others, 2000). This hypothesis could be investigated by video monitoring at bird nests to document adult and nestling failure and causes of failure (Ellison and Ribic, 2012).
At elevated local lead concentrations in soil (4,400 ppm), period nest survival was only 2 to 14 percent for three of the four open-cup nesting bird species (eastern towhee, indigo bunting, northern cardinal; fig. 17). Although field sparrows showed an inverse relation in which nest success increased slightly as lead concentrations in soil increased, support for this was minimal because the credible interval for this effect overlapped zero, and the effect had only a 0.69 probability of being positive. Donovan and Thompson (2001) used a simple population model to demonstrate that a typical migrant songbird with 60 to 70 percent adult survival would likely exhibit population decline if period nest survival was below 20 to 30 percent. Although other demographic parameters, such as number of nesting attempts and the propensity to double brood, need to be factored into a population model, it is possible that birds occupying areas with elevated local lead concentrations are not producing enough young to balance adult mortality and sustain a local population. Nevertheless, birds may persist at a site despite low reproduction if the local population is supported by immigrants from other areas, creating a population sink. Although sink populations can persist under certain scenarios, they can have consequences for the larger metapopulation and even create regional declines (Pulliam, 1988; Donovan and others, 1995). Eastern bluebird, eastern towhee, field sparrow, indigo bunting, and northern cardinal are all exhibiting population declines of 0.19 to 2.28 percent per year in the Central Hardwood Region (not shown), which encompasses our study sites (Sauer and others, 2017).
Nestling growth and fledgling weight, which affect survival after fledging, may also be sensitive indicators of environmental conditions and are therefore potential alternative measures of reproductive performance (Furness and Greenwood, 1993). Although nestlings in the present study were weighed and measured before fledging, their ages varied or were unknown. However, future analyses could assess body condition as the residuals from a regression of weight on tarsus or wing chord and relate this to lead concentrations. Moreover, reproductive success was based on a sample of birds that started nesting. It is possible that the individual birds that were the most severely affected by lead exposure and uptake died before having the opportunity to breed; alternately, the affected birds did not breed as a direct result of their exposure or left the area without breeding. This may have reduced the frequency with which lead could be observed to affect the reproductive outcomes that we measured. In addition, this study focused on breeding adults and did not account for lead effects on nonbreeding individuals inhabiting the study sites. Additional analyses of cumulative effects, such as annual reproductive success using species-specific information (in contrast to the daily nest survival metric used here), may be warranted.
Conclusions
The results of this large-scale multiyear investigation provide evidence that lead exposure and uptake have occurred in adult birds and their offspring at contaminated sites within the Southeast Missouri Lead Mining District. Birds at contaminated sites generally had greater lead concentrations in blood, liver, and kidney tissues compared to those at reference sites; also, lead concentrations in blood tended to increase as lead concentrations in soil increased. This correlation indicates that the lead in soils (via direct or indirect ingestion of soil and dusts) and the food web (that is, invertebrates) at the study sites was bioavailable to birds. Moreover, the results for lead in blood, particularly blood collected from nestlings, indicate that the lead exposures were local to the breeding territories. Variations in lead concentrations in blood were likely affected by soil heterogeneity at the contaminated sites and by the rates of ingestion of lead-contaminated soils.
Also, elevated lead concentrations in tissues were negatively correlated with δALAD activity and positively correlated with indicators of oxidative stress, potentially indicating additional sublethal effects of lead on birds at the contaminated study sites relative to the reference sites. In total, 53 percent of all birds and 50 percent of northern cardinals from contaminated sites had δALAD activity <50 percent of the reference sites; about 40 to 60 percent of birds at the contaminated sites, compared to less than or equal to 5 percent at the reference sites, had lead concentrations in their blood, liver, and (or) kidney tissues that exceeded literature-based adverse effects concentrations.
Some evidence indicates that lead in soil, and subsequently in blood, have negatively affected reproductive outcomes for several species. Impaired reproduction related to lead exposure was most strongly supported for nest success in eastern towhees and indigo buntings, and it was marginally supported for northern cardinals. Notably, at elevated local lead concentrations in soil (4,400 ppm), period nest survival was only 2 to 14 percent for eastern towhees, indigo buntings and northern cardinals; these levels of nest survival (<20 percent) are not likely able to sustain a local population. There were weakly supported or suggestive negative relations between local lead concentration in soil, clutch size, and hatching success in eastern bluebirds and number fledged in field sparrows and eastern towhee. In addition, means for clutch size, proportion hatched, number of young fledged, and number of young fledged by successful nests were greater for reference sites than contaminated sites in 17 of 20 comparisons. Ultimately, these results indicate that soil-borne lead contamination in the Southeast Missouri Lead Mining District has adversely affected birds. These results may help land and resource managers determine strategies needed to reduce lead concentrations in soil and invertebrates and to mitigate aeolian transport of lead-contaminated soils.
References Cited
Aelion, C.M., Davis, H.T., McDermott, S., and Lawson, A.B., 2008, Metals concentrations in rural topsoil in South Carolina—Potential for human health impact: Science of the Total Environment, v. 402, no. 2–3, p. 149–156, accessed June 2022 at https://doi.org/10.1016/j.scitotenv.2008.04.043.
Ahamed, M., and Siddiqui, M.K.J., 2007, Low level lead exposure and oxidative stress—Current opinions: Clinica Chimica Acta, v. 383, no. 1–2, p. 57–64, accessed October 2019 at https://doi.org/10.1016/j.cca.2007.04.024.
Allert, A.L., Fairchild, J.F., DiStefano, R.J., Schmitt, C.J., Brumbaugh, W.G., and Besser, J.M., 2009, Ecological effects of lead mining on Ozark streams—In-situ toxicity to woodland crayfish (Orconectes hylas): Ecotoxicology and Environmental Safety, v. 72, no. 4, p. 1207–1219, accessed September 2021 at https://doi.org/10.1016/j.ecoenv.2008.08.005.
Allert, A.L., DiStefano, R.J., Fairchild, J.F., Schmitt, C.J., McKee, M.J., Girondo, J.A., Brumbaugh, W.G., and May, T.W., 2013, Effects of historical lead-zinc mining on riffle-dwelling benthic fish and crayfish in the Big River of southeastern Missouri, USA: Ecotoxicology (London, England), v. 22, no. 3, p. 506–521, accessed April 2020 at https://doi.org/10.1007/s10646-013-1043-3.
Ayala, A., Munoz, M.F., and Arguelles, S., 2014, Lipid peroxidation—Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal: Oxidative Medicine and Cellular Longevity, v. 2014, no. 6, 31 p., accessed October 2019 at https://doi.org/10.1155/2014/360438.
Barltrop, D., and Meek, F., 1975, Absorption of different lead compounds: Postgraduate Medical Journal, v. 51, no. 601, p. 805–809, accessed June 2022 at https://doi.org/10.1136/pgmj.51.601.805.
Berglund, Å.M.M., Koivula, M.J., and Eeva, T., 2011, Species- and age-related variation in metal exposure and accumulation of two passerine bird species: Environmental Pollution, v. 159, no. 10, p. 2368–2374, accessed June 2019 at https://doi.org/10.1016/j.envpol.2011.07.001.
Besser, J.M., Brumbaugh, W.G., May, T.W., and Schmitt, C.J., 2007, Biomonitoring of lead, zinc, and cadmium in streams draining lead-mining and non-mining areas, Southeast Missouri, USA: Environmental Monitoring and Assessment, v. 129, no. 1–3, p. 227–241, accessed April 2020 at https://doi.org/10.1007/s10661-006-9356-9.
Beyer, W.N., Basta, N.T., Chaney, R.L., Henry, P.F., Mosby, D.E., Rattner, B.A., Scheckel, K.G., Sprague, D.T., and Weber, J.S., 2016, Bioaccessibility tests accurately estimate bioavailability of lead to quail: Environmental Toxicology and Chemistry, v. 35, no. 9, p. 2311–2319, accessed June 2019 at https://doi.org/10.1002/etc.3399.
Beyer, W.N., Casteel, S.W., Friedrichs, K.R., Gramlich, E., Houseright, R.A., Nichols, J.R., Karouna-Renier, N.K., Kim, D.Y., Rangen, K.L., Rattner, B.A., and Schultz, S.L., 2018, Biomarker responses of Peromyscus leucopus exposed to lead and cadmium in the Southeast Missouri Lead Mining District: Environmental Monitoring and Assessment, v. 190, no. 104, p. 1–16, accessed April 2020 at https://doi.org/10.1007/s10661-017-6442-0.
Beyer, W.N., Connor, E.E., and Gerould, S., 1994, Estimates of soil ingestion by wildlife: The Journal of Wildlife Management, v. 58, no. 2, p. 375–382, accessed October 2019 at https://doi.org/10.2307/3809405.
Beyer, W.N., Dalgarn, J., Dudding, S., French, J.B., Mateo, R., Miesner, J., Sileo, L., and Spann, J., 2004, Zinc and lead poisoning in wild birds in the Tri-State Mining District (Oklahoma, Kansas, and Missouri): Archives of Environmental Contamination and Toxicology, v. 48, no. 1, p. 108–117, accessed June 2019 at https://doi.org/10.1007/s00244-004-0010-7.
Beyer, W.N., Franson, J.C., French, J.B., May, T., Rattner, B.A., Shearn-Bochsler, V.I., Warner, S.E., Weber, J., and Mosby, D., 2013, Toxic exposure of songbirds to lead in the Southeast Missouri Lead Mining District: Archives of Environmental Contamination and Toxicology, v. 65, no. 3, p. 598–610, accessed September 2019 at https://doi.org/10.1007/s00244-013-9923-3.
Beyer, W.N., and Sample, B.E., 2017, An evaluation of inorganic toxicity reference values for use in assessing hazards to American robins (Turdus migratorius): Integrated Environmental Assessment and Management, v. 13, no. 2, p. 352–359. [Also available at https://doi.org/10.1002/ieam.1792.]
Beyer, W.N., Spann, J.W., Sileo, L., and Franson, J.C., 1988, Lead poisoning in six captive avian species: Archives of Environmental Contamination and Toxicology, v. 17, p. 121–130, accessed September 2021 at https://doi.org/10.1007/BF01055162.
Blus, L.J., Henny, C.J., Hoffman, D.J., and Grove, R.A., 1995, Accumulation in and effects of lead and cadmium on waterfowl and passerines in northern Idaho: Environmental Pollution, v. 89, no. 3, p. 311–318, accessed July 2019 at https://doi.org/10.1016/0269-7491(94)00069-P.
Brambor, T., Clark, W.R., and Golder, M., 2006, Understanding interaction models—Improving empirical analyses: Political Analysis, v. 14, no. 1, p. 63–82, accessed September 2021 at https://doi.org/10.1093/pan/mpi014.
Brumbaugh, W.G., Schmitt, C.J., and May, T.W., 2005, Concentrations of cadmium, lead, and zinc in fish from mining-influenced waters of northeastern Oklahoma—Sampling of blood, carcass, and liver for aquatic biomonitoring: Archives of Environmental Contamination and Toxicology, v. 49, no. 1, p. 76–88, accessed April 2020 at https://doi.org/10.1007/s00244-004-0172-3.
Buekers, J., Steen Redeker, E., and Smolders, E., 2009, Lead toxicity to wildlife—Derivation of a critical blood concentration for wildlife monitoring based on literature data: Science of the Total Environment, v. 407, no. 11, p. 3431–3438, accessed July 2019 at https://doi.org/10.1016/j.scitotenv.2009.01.044.
Buerger, T.T., Mirarchi, R.E., and Lisano, M.E., 1986, Effects of lead shot ingestion on captive mourning dove survivability and reproduction: The Journal of Wildlife Management, v. 50, no. 1, p. 1–8, accessed July 2019 at https://doi.org/10.2307/3801479.
Burch, H.B., and Siegel, A.L., 1971, Improved method for measurement of delta-aminolevulinic acid dehydratase activity in human erythrocytes: Clinical Chemistry, v. 17, no. 10, p. 1038–1041, accessed April 2020 at https://doi.org/10.1093/clinchem/17.10.1038.
Burford, J., 1978, Underground treasures: The story of mining in Missouri, in Johnson, K.M., ed., Official Manual State of Missouri 1977–1978: Jefferson City, Mo., p. 1–33, accessed January 2023 at https://mdh.contentdm.oclc.org/digital/api/collection/bluebook/id/47545/page/0/inline/bluebook_47545_0.
Burger, J., 1995, A risk assessment for lead in birds: Journal of Toxicology and Environmental Health, v. 45, no. 4, p. 369–396, accessed July 2019 at https://doi.org/10.1080/15287399509532003.
Burger, J., 1998, Effects of lead on sibling recognition in young herring gulls: Toxicological Sciences, v. 43, no. 2, p. 155–160, accessed July 2019 at https://doi.org/10.1006/toxs.1998.2451.
Burger, J., and Gochfeld, M., 1985, Early postnatal lead exposure—Behavioral effects in common tern chicks (Sterna hirundo): Journal of Toxicology and Environmental Health, v. 16, no. 6, p. 869–886, accessed September 2021 at https://doi.org/10.1080/15287398509530794.
Burger, J., and Gochfeld, M., 1991, Cadmium and lead in common terns (Aves—Sterna hirundo)—Relationship between levels in parents and eggs: Environmental Monitoring and Assessment, v. 16, p. 253–258, accessed April 2020 at https://doi.org/10.1007/BF00397612.
Burger, J., and Gochfeld, M., 1993, Lead and cadmium accumulation in eggs and fledgling seabirds in the New York bight: Environmental Toxicology and Chemistry, v. 12, no. 2, p. 261–267, accessed September 2021 at https://doi.org/10.1002/etc.5620120209.
Burger, J., and Gochfeld, M., 1996, Lead and behavioral development—Parental compensation for behaviorally impaired chicks: Pharmacology, Biochemistry, and Behavior, v. 55, no. 3, p. 339–349, accessed September 2021 at https://doi.org/10.1016/S0091-3057(96)00103-7.
Burger, J., and Gochfeld, M., 1997, Age differences in metals in the blood of herring (Larus argentatus) and Franklin’s (Larus pipixcan) gulls: Archives of Environmental Contamination and Toxicology, v. 33, no. 4, p. 436–440. [Also available at https://doi.org/10.1007/s002449900274.]
Burger, J., and Gochfeld, M., 2000, Effects of lead on birds (Laridae)—A review of laboratory and field studies: Journal of Toxicology and Environmental Health. Part B, Critical Reviews, v. 3, no. 2, p. 59–78, accessed April 2020 at https://doi.org/10.1080/109374000281096.
Burger, J., and Gochfeld, M., 2005, Effects of lead on learning in herring gulls—An avian wildlife model for neurobehavioral deficits: Neurotoxicology, v. 26, no. 4, p. 615–624, accessed September 2021 at https://doi.org/10.1016/j.neuro.2005.01.005.
Burhans, D.E., Dearborn, D., Thompson, F.R., III, and Faaborg, J., 2002, Factors affecting predation at songbird nests in old fields: The Journal of Wildlife Management, v. 66, no. 1, p. 240–249, accessed June 2022 at https://doi.org/10.2307/3802890.
Carey, M., Burhans, D.E., and Nelson, D.A., 2008, Field sparrow (Spizella pusilla) (ver. 2.0), in Poole, A.F., ed., The birds of North America: Ithaca, N.Y., Cornell Lab of Ornithology, accessed October 2019 at https://doi.org/10.2173/bna.103.
Casteel, S.W., Weis, C.P., Henningsen, G.M., and Brattin, W.J., 2006, Estimation of relative bioavailability of lead in soil and soil-like materials using young swine: Environmental Health Perspectives, v. 114, no. 8, p. 1162–1171, accessed September 2019 at https://doi.org/10.1289/ehp.8852.
Cleveland, D.M., Rattner, B.A., Karouna-Renier, N.K., and Lankton, J.S., 2023, Breeding songbird tissue analysis and metal concentrations in tissues, soil and invertebrates collected near nesting sites within the Southeast Missouri Lead Mining District, 2016–19: U.S. Geological Survey data release, https://doi.org/10.5066/P9RV1D60.
Conway, C.J., and Martin, T.E., 2000, Evolution of passerine incubation behavior—Influence of food, temperature, and nest predation: Evolution; International Journal of Organic Evolution, v. 54, no. 2, p. 670–685, accessed October 2019 at https://doi.org/10.1111/j.0014-3820.2000.tb00068.x.
Cram, D.L., Blount, J.D., York, J.E., and Young, A.J., 2015, Immune response in a wild bird is predicted by oxidative status, but does not cause oxidative stress: PLoS ONE, v. 10, no. 3, 14 p., accessed June 2022 at https://doi.org/10.1371/journal.pone.0122421.
Custer, T.W., Franson, J.C., and Pattee, O.H., 1984, Tissue lead distribution and hematologic effects in American kestrels (Falco sparverius L.) fed biologically incorporated lead: Journal of Wildlife Diseases, v. 20, no. 1, p. 39–43, accessed April 2020 at https://doi.org/10.7589/0090-3558-20.1.39.
Custer, C., Custer, T., Archuleta, A., Coppock, L., Swartz, C., and Bickham, J., 2003, A mining impacted stream—Exposure and effects of lead and other trace elements on tree swallows (Tachycineta bicolor) nesting in the upper Arkansas River Basin, Colorado, chap. 28 of Hoffman, D.J., Rattner, B.A., Burton, G.A., Jr., and Carins, J., Jr., eds., Handbook of ecotoxicology (2d ed.): Boca Raton, Fla., Lewis Publishers, p. 787–812. [Also available at https://doi.org/10.1201/9781420032505.]
De Francisco, N., Ruiz Troya, J.D., and Agüera, E.I., 2003, Lead and lead toxicity in domestic and free living birds: Avian Pathology, v. 32, no. 1, p. 3–13, accessed April 2020 at https://doi.org/10.1080/0307945021000070660.
Del Bono, G., and Braca, G., 1973, Lead poisoning in domestic and wild ducks: Avian Pathology, v. 2, no. 3, p. 195–209, accessed April 2020 at https://doi.org/10.1080/03079457309353796.
DeMichele, S.J., 1984, Nutrition of lead: Comparative Biochemistry and Physiology, Part A—Physiology, v. 78, no. 3, p. 401–408, accessed April 2020 at https://doi.org/10.1016/0300-9629(84)90567-X.
Dieter, M.P., and Finley, M.T., 1979, δ-aminolevulinic acid dehydratase enzyme activity in blood, brain, and liver of lead-dosed ducks: Environmental Research, v. 19, no. 1, p. 127–135, accessed June 2022 at https://doi.org/10.1016/0013-9351(79)90041-0.
Dinsmore, S.J., White, G.W., and Knopf, F.L., 2002, Advanced techniques for modeling avian nest survival: Ecology, v. 83, no. 12, p. 3476–3488, accessed April 2020 at https://doi.org/10.1890/0012-9658(2002)083[3476:ATFMAN]2.0.CO;2.
Donovan, T.M., Lamberson, R.H., Kimber, A., Thompson, F.R., III, and Faaborg, J., 1995, Modeling the effects of habitat fragmentation on source and sink demography of neotropical migrant birds: Conservation Biology, v. 9, no. 6, p. 1396–1407, accessed August 2020 at https://doi.org/10.1046/j.1523-1739.1995.09061396.x.
Donovan, T.M., and Thompson, F.R., III, 2001, Modeling the ecological trap hypothesis—A habitat and demographic analysis for migrant songbirds: Ecological Applications, v. 11, no. 3, p. 871–882, accessed October 2019 at https://doi.org/10.2307/3061122.
Ecke, F., Singh, N.J., Arnemo, J.M., Bignert, A., Helander, B., Berglund, A.M.M., Borg, H., Bröjer, C., Holm, K., Lanzone, M., Miller, T., Nordström, Å., Räikkönen, J., Rodushkin, I., Ågren, E., and Hörnfeldt, B., 2017, Sublethal lead exposure alters movement behavior in free-ranging golden eagles: Environmental Science & Technology, v. 51, no. 10, p. 5729–5736, accessed September 2021 at https://doi.org/10.1021/acs.est.6b06024.
Edens, F.W., Benton, E., Bursian, S.J., and Morgan, G.W., 1976, Effect of dietary lead on reproductive performance in Japanese quail, Coturnix coturnix japonica: Toxicology and Applied Pharmacology, v. 38, no. 2, p. 307–314, accessed September 2019 at https://doi.org/10.1016/0041-008X(76)90137-X.
Edens, F.W., and Garlich, J.D., 1983, Lead-induced egg production decrease in leghorn and Japanese quail hens: Poultry Science, v. 62, no. 9, p. 1757–1763, accessed September 2019 at https://doi.org/10.3382/ps.0621757.
Eisler, R., 1988, Lead hazards to fish, wildlife, and invertebrates—A synoptic review: U.S. Fish and Wildlife Service, Biological Report 85(1.14), Contaminant Hazard Reviews Report no. 14, 94 p., accessed April 2020 at https://www.pwrc.usgs.gov/eisler/CHR_14_Lead.pdf.
Ellison, K.S., and Ribic, C.A., 2012, Nest defense—Grassland bird responses to snakes, chap. 12 of Ribic, C.A., Thompson, F.R., III, and Pietz, P.J., eds., Video surveillance of nesting birds: Berkeley, Calif., University of California Press, Studies in Avian Biology, no. 43, p. 149–160, accessed October 2019 at https://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1252&context=usgsnpwrc.
Espín, S., Martínez-López, E., Jiménez, P., María-Mojica, P., and García-Fernández, A.J., 2015, Delta-aminolevulinic acid dehydratase (δALAD) activity in four free-living bird species exposed to different levels of lead under natural conditions: Environmental Research, v. 137, p. 185–198, accessed April 2020 at https://doi.org/10.1016/j.envres.2014.12.017.
Fair, J.M., Paul, E., and Jones, J., eds., 2010, Guidelines to the use of wild birds in research: Washington, D.C., The Ornithological Council, 215 p., accessed October 2019 at https://birdnet.org/wp-content/uploads/2022/09/guidelines_august2010.pdf.
Fair, J.M., and Myers, O.B., 2002, The ecological and physiological costs of lead shot and immunological challenge to developing Western Bluebirds: Ecotoxicology (London, England), v. 11, p. 199–208, accessed April 2020 at https://doi.org/10.1023/A:1015474832239.
Fallon, J.A., Redig, P., Miller, T.A., Lanzone, M., and Katzner, T., 2017, Guidelines for evaluation and treatment of lead poisoning of wild raptors: Wildlife Society Bulletin, v. 41, no. 2, p. 205–211, accessed June 2022 at https://doi.org/10.1002/wsb.762.
Finley, M.T., and Dieter, M.P., 1978, Erythrocyte δ-aminolevulinic acid dehydratase activity in mallard ducks—Duration of inhibition after lead shot dosage: The Journal of Wildlife Management, v. 42, no. 3, p. 621–625, accessed August 2020 at https://doi.org/10.2307/3800826.
Fisher, I.J., Pain, D.J., and Thomas, V.G., 2006, A review of lead poisoning from ammunition sources in terrestrial birds: Biological Conservation, v. 131, no. 3, p. 421–432, accessed April 2020 at https://doi.org/10.1016/j.biocon.2006.02.018.
Franson, J.C., and Pain, D.J., 2011, Lead in birds, in Beyer, W.N., and Meador, J.P., eds., Environmental contaminants in biota—Interpreting tissue concentrations (2d ed.): Boca Raton, Fla., CRC Press, p. 563–594., accessed October 2019 at https://doi.org/10.1201/b10598-17.
Franson, J.C., and Russell, R.E., 2014, Lead and eagles—Demographic and pathological characteristics of poisoning, and exposure levels associated with other causes of mortality: Ecotoxicology (London, England), v. 23, no. 9, p. 1722–1731, accessed September 2019 at https://doi.org/10.1007/s10646-014-1337-0.
Friedman, S.L., Brasso, R.L., and Condon, A.M., 2008, An improved, simple nest-box trap: Journal of Field Ornithology, v. 79, no. 1, p. 99–101, accessed October 2019 at https://doi.org/10.1111/j.1557-9263.2008.00150.x.
Friedrich, S., Konietschke, F., and Pauly, M., 2019, Resampling-based analysis of multivariate data and repeated measures designs with the R Package MANOVA.RM: The R Journal, v. 11, no. 2, accessed October 2019 at https://doi.org/10.32614/RJ-2019-051.
Furness, R.W., and Greenwood, J.J.D., eds., 1993, Birds as monitors of environmental change (1st. ed.): Netherlands, Springer Dordrecht, 356 p. [Also available at https://doi.org/10.1007/978-94-015-1322-7.]
Gale, N.L., Adams, C.D., Wixson, B.G., Loftin, K.A., and Huang, Y.-W., 2004, Lead, zinc, copper, and cadmium in fish and sediments from the Big River and Flat River Creek of Missouri’s old lead belt: Environmental Geochemistry and Health, v. 26, p. 37–49, accessed June 2022 at https://doi.org/10.1023/B:EGAH.0000020935.89794.57.
Gaston, K.J., 2022, Birds and ecosystem services: Current Biology, v. 32, no. 20, p. R1163–R1166, accessed February 2023 at https://doi.org/10.1016/j.cub.2022.07.053.
Gómez-Ramírez, P., Martínez-López, E., María-Mojica, P., León-Ortega, M., and García-Fernández, A.J., 2011, Blood lead levels and δ-ALAD inhibition in nestlings of Eurasian eagle owl (Bubo bubo) to assess lead exposure associated to an abandoned mining area: Ecotoxicology (London, England), v. 20, p. 131–138, accessed September 2019 at https://doi.org/10.1007/s10646-010-0563-3.
Goodchild, C.G., Beck, M.L., VanDiest, I., Czesak, F.N., Lane, S.J., and Sewall, K.B., 2021, Male zebra finches exposed to lead (Pb) during development have reduced volume of song nuclei, altered sexual traits, and received less attention from females as adults: Ecotoxicology and Environmental Safety, v. 210, 111850 p., accessed June 2022 at https://doi.org/10.1016/j.ecoenv.2020.111850.
Gowaty, P.A., and Plissner, J.H., 2015, Eastern bluebird (Sialia sialis) (ver. 2.0), in Poole, A.F., ed., The birds of North America: Ithaca, N.Y., Cornell Lab of Ornithology, accessed April 2020 at https://doi.org/10.2173/bna.381.
Greenlaw, J.S., 2015, Eastern towhee (Pipilo erythrophthalmus) (ver. 2.0), in Rodewald, P.G., ed., The birds of North America: Ithaca, N.Y., Cornell Lab of Ornithology, accessed October 2019 at https://doi.org/10.2173/bna.262.
Grue, C.E., O’Shea, T.J., and Hoffman, D.J., 1984, Lead concentrations and reproduction in highway-nesting barn swallows: The Condor, v. 86, no. 4, p. 383–389, accessed September 2019 at https://doi.org/10.2307/1366811.
Grue, C.E., Hoffman, D.J., Nelson Beyer, W., and Franson, L.P., 1986, Lead concentrations and reproductive success in European starlings Sturnus vulgaris nesting within highway roadside verges: Environmental Pollution Series A, Ecological and Biological, v. 42, no. 2, p. 157–182, accessed September 2019 at https://doi.org/10.1016/0143-1471(86)90005-X.
Guan, X., 2010, Genomic and biochemical analysis of oxidative stress in birds with diverse longevities: Blacksburg, Va., Virginia Polytechnic Institute and State University, Ph. D. dissertation, 170 p., accessed October 2019 at https://vtechworks.lib.vt.edu/handle/10919/27827.
Haig, S.M., D’Elia, J., Eagles-Smith, C., Fair, J.M., Gervais, J., Herring, G., Rivers, J.W., and Schulz, J.H., 2014, The persistent problem of lead poisoning in birds from ammunition and fishing tackle: The Condor, v. 116, no. 3, p. 408–428, accessed October 2019 at https://doi.org/10.1650/CONDOR-14-36.1.
Hansen, J.A., Audet, D., Spears, B.L., Healy, K.A., Brazzle, R.E., Hoffman, D.J., Dailey, A., and Beyer, W.N., 2011, Lead exposure and poisoning of songbirds using the Coeur d’Alene River Basin, Idaho, USA: Integrated Environmental Assessment and Management, v. 7, no. 4, p. 587–595, accessed June 2019 at https://doi.org/10.1002/ieam.201.
Halkin, S.L., and Linville, S.U., 1999, Northern cardinal (Cardinalis cardinalis) (ver. 1.0), in Poole, A.F., and Gill, F.B., eds., The birds of North America: Cornell Lab of Ornithology, accessed October 2019 at https://doi.org/10.2173/bna.440.
Haschek, W.M., Rousseaux, C.G., and Wallig, M.A., 2013, Safety assessment in toxicologic pathology—An introduction, vol. II of Haschek, W.M., Rousseaux, C.G., Wallig, M.A., Bolon, B., Ochoa, R., and Mahler, B.W., eds., Haschek and Rousseaux’s handbook of toxicologic pathology (3d ed.): London, United Kingdom, Elsevier, 3,054 p. [Also available at https://doi.org/10.1016/C2010-1-67850-9.]
Hemphill, C.P., Ruby, M.V., Beck, B.D., Davis, A., and Bergstrom, P.D., 1991, The bioavailability of lead in mining wastes—Physical/chemical considerations: Chemical Speciation and Bioavailability, v. 3, no. 3–4, p. 135–148, accessed September 2019 at https://doi.org/10.1080/09542299.1991.11083165.
Hettiarachchi, G.M., and Pierzynski, G.M., 2004, Soil lead bioavailability and in situ remediation of lead-contaminated soils—A review: Environmental Progress, v. 23, no. 1, p. 78–93, accessed August 2019 at https://doi.org/10.1002/ep.10004.
Hoffman, D.J., Franson, J.C., Pattee, O.H., Bunck, C.M., and Anderson, A., 1985a, Survival, growth, and accumulation of ingested lead in nestling American kestrels (Falco sparverius): Archives of Environmental Contamination and Toxicology, v. 14, no. 1, p. 89–94, accessed July 2019 at https://doi.org/10.1007/BF01055766.
Hoffman, D.J., Christian Franson, J., Pattee, O.H., Bunck, C.M., and Murray, H.C., 1985b, Biochemical and hematological effects of lead ingestion in nestling American kestrels (Falco sparverius): Comparative Biochemistry and Physiology Part C—Comparative Pharmacology and Toxicology, v. 80, no. 2, p. 431–439, accessed July 2019 at https://doi.org/10.1016/0742-8413(85)90080-5.
Hoffman, D.J., Heinz, G.H., Sileo, L., Audet, D.J., Campbell, J.K., and Obrecht, H.H., III, 2000, Developmental toxicity of lead-contaminated sediment in Canada geese (Branta canadensis): Journal of Toxicology and Environmental Health—Part A., v. 59, no. 4, p. 235–252, accessed April 2020 at https://doi.org/10.1080/009841000156916.
Horton, J.D., 2017, The State Geologic Map Compilation (SGMC) geodatabase of the conterminous United States (ver. 1.1, August 2017): U.S. Geological Survey data release, accessed September 2021 at https://doi.org/10.5066/F7WH2N65.
Howell, J.C., 1942, Notes on the nesting habits of the American robin (Turdus migratorius L.): American Midland Naturalist, v. 28, no. 3, p. 529–603, accessed April 2020 at https://www.jstor.org/stable/2420891.
Isaksson, C., Andersson, M.N., Nord, A., von Post, M., and Wang, H.-L., 2017, Species-dependent effects of the urban environment on fatty acid composition and oxidative stress in birds: Frontiers in Ecology and Evolution, v. 5, no. 44, accessed October 2019 at https://doi.org/10.3389/fevo.2017.00044.
Janssens, E., Dauwe, T., Pinxten, R., Bervoets, L., Blust, R., and Eens, M., 2003, Effects of heavy metal exposure on the condition and health of nestlings of the great tit (Parus major)—A small songbird species: Environmental Pollution, v. 126, no. 2, p. 267–274, accessed September 2019 at https://doi.org/10.1016/S0269-7491(03)00185-4.
Johnson, G.D., Audet, D.J., Kern, J.W., LeCaptain, L.J., Strickland, M.D., Hoffman, D.J., and McDonald, L.L., 1999, Lead exposure in passerines inhabiting lead-contaminated floodplains in the Coeur d’Alene River Basin, Idaho, USA: Environmental Toxicology and Chemistry, v. 18, no. 6, p. 1190–1194, accessed April 2020 at https://doi.org/10.1002/etc.5620180617.
Johnson, M.S., Wickwire, W.T., Quinn, M.J., Jr., Ziolkowski, D.J., Jr., Burmistrov, D., Menzie, C.A., Geraghty, C., Minnich, M., and Parsons, P.J., 2007, Are songbirds at risk from lead at small arms ranges? An application of the spatially explicit exposure model: Environmental Toxicology and Chemistry, v. 26, no. 10, p. 2215–2225, accessed October 2019 at https://doi.org/10.1897/07-068R.1.
Johnson, D.H., 1979, Estimating nest success—The Mayfield method and an alternative: The Auk, v. 96, no. 4, p. 651–661, accessed April 2020 at https://www.jstor.org/stable/4085651.
Kastury, F., Smith, E., Lombi, E., Donnelley, M.W., Cmielewski, P.L., Parsons, D.W., Noerpel, M., Scheckel, K.G., Kingston, A.M., Myers, G.R., Paterson, D., de Jonge, M.D., and Juhasz, A.L., 2019, Dynamics of lead bioavailability and speciation in indoor dust and X-ray spectroscopic investigation of the link between ingestion and inhalation pathways: Environmental Science & Technology, v. 53, no. 19, p. 11486–11495, accessed September 2021 at https://doi.org/10.1021/acs.est.9b03249.
Kendall, R.J., and Scanlon, P.F., 1981, Effects of chronic lead ingestion on reproductive characteristics of ringed turtle doves Streptopelia risoria and on tissue lead concentrations of adults and their progeny: Environmental Pollution. Series A. Ecological and Biological, v. 26, no. 3, p. 203–213, accessed April 2020 at https://doi.org/10.1016/0143-1471(81)90006-4.
Kendall, R.J., Scanlon, P.F., and Veit, H.P., 1983, Histologic and ultrastructural lesions of mourning doves (Zenaida macroura) poisoned by lead shot: Poultry Science, v. 62, no. 6, p. 952–956, accessed June 2022 at https://doi.org/10.3382/ps.0620952.
Kendall, R.J., Scanlon, P.F., and Di Giulio, R.T., 1982, Toxicology of ingested lead shot in ringed turtle doves: Archives of Environmental Contamination and Toxicology, v. 11, p. 259–263, accessed June 2022 at https://doi.org/10.1007/BF01055200.
Kéry, M., and Schaub, M., 2012, Bayesian population analysis using WinBUGS—A hierarchical perspective: Waltham, Mass., Academic Press, 554 p. [Also available at https://doi.org/10.1016/C2010-0-68368-4.]
Koivula, M.J., and Eeva, T., 2010, Metal-related oxidative stress in birds: Environmental Pollution, v. 158, no. 7, p. 2359–2370, accessed June 2022 at https://doi.org/10.1016/j.envpol.2010.03.013.
Leniowski, K., and Węgrzyn, E., 2018, Synchronisation of parental behaviours reduces the risk of nest predation in a socially monogamous passerine bird: Scientific Reports, v. 8, no. 7385, 9 p., accessed October 2019 at https://doi.org/10.1038/s41598-018-25746-5.
Liu, C.-M., Ma, J.-Q., and Sun, Y.-Z., 2012, Puerarin protects the rat liver against oxidative stress-mediated DNA damage and apoptosis induced by lead: Experimental and Toxicologic Pathology, v. 64, no. 6, p. 575–582, accessed October 2019 at https://doi.org/10.1016/j.etp.2010.11.016.
Lowther, P.E., 2020, Brown-headed cowbird (Molothrus ater) (ver. 1.0), in Poole, A.F., and Gill, F.B., eds., Birds of the world: Ithaca, N.Y., Cornell Lab of Ornithology, accessed September 2021 at https://doi.org/10.2173/bow.bnhcow.01.
Maedgen, J.L., Hacker, C.S., Schroder, G.D., and Weir, F.W., 1982, Bioaccumulation of lead and cadmium in the royal tern and sandwich tern: Archives of Environmental Contamination and Toxicology, v. 11, p. 99–102, accessed September 2021 at https://doi.org/10.1007/BF01055193.
Magrath, R.D., 1991, Nestling weight and juvenile survival in the blackbird, Turdus merula: Journal of Animal Ecology, v. 60, no. 1, p. 335–351, accessed April 2020 at https://doi.org/10.2307/5464.
Martin, T.E., Scott, J., and Menge, C., 2000, Nest predation increases with parental activity—Separating nest site and parental activity effects: Proceedings—Biological Sciences, v. 267, no. 1459, p. 2287–2293, accessed October 2019 at https://doi.org/10.1098/rspb.2000.1281.
Mateo, R., Beyer, W.N., Spann, J.W., Hoffman, D.J., and Ramis, A., 2003, Relationship between oxidative stress, pathology, and behavioral signs in lead poisoning in mallards: Journal of Toxicology and Environmental Health—Part A, v. 66, no. 14, p. 1371–1389, accessed October 2019 at https://doi.org/10.1080/15287390306390.
Mateo, R., and Hoffman, D.J., 2001, Differences in oxidative stress between young Canada geese and mallards exposed to lead-contaminated sediment: Journal of Toxicology and Environmental Health—Part A, v. 64, no. 7, p. 531–545, accessed April 2020 at https://doi.org/10.1080/15287390152627228.
Matović, V., Buha, A., Đukić-Ćosić, D., and Bulat, Z., 2015, Insight into the oxidative stress induced by lead and/or cadmium in blood, liver, and kidneys: Food and Chemical Toxicology, v. 78, p. 130–140, accessed September 2021 at https://doi.org/10.1016/j.fct.2015.02.011.
Mayfield, H.F., 1961, Nesting success calculated from exposure: The Wilson Bulletin, v. 73, no. 3, p. 255–261, accessed April 2020 at https://www.jstor.org/stable/4158936.
Mayfield, H.F., 1975, Suggestions for calculating nest success: The Wilson Bulletin, v. 87, no. 4, p. 456–466, accessed June 2022 at https://www.jstor.org/stable/4160682.
McClelland, S.C., Durães Ribeiro, R., Mielke, H.W., Finkelstein, M.E., Gonzales, C.R., Jones, J.A., Komdeur, J., Derryberry, E., Saltzberg, E.B., and Karubian, J., 2019, Sub-lethal exposure to lead is associated with heightened aggression in an urban songbird: Science of the Total Environment, v. 654, p. 593–603, accessed June 2022 at https://doi.org/10.1016/j.scitotenv.2018.11.145.
Ming, H., He, W.-X., Lamb, D.T., Megharaj, M., and Naidu, R., 2012, Bioavailability of lead in contaminated soil depends on the nature of bioreceptor: Ecotoxicology and Environmental Safety, v. 78, p. 344–350, accessed September 2021 at https://doi.org/10.1016/j.ecoenv.2011.11.045.
Minias, P., 2015, The use of haemoglobin concentrations to assess physiological condition in birds—A review: Conservation Physiology, v. 3, no. 1, 15 p., accessed June 2022 at https://doi.org/10.1093/conphys/cov007.
Monaghan, P., Metcalfe, N.B., and Torres, R., 2009, Oxidative stress as a mediator of life history trade-offs—Mechanisms, measurements and interpretation: Ecology Letters, v. 12, no. 1, p. 75–92, accessed October 2019 at https://doi.org/10.1111/j.1461-0248.2008.01258.x.
Monrós, J.S., Belda, E.J., and Barba, E., 2002, Post-fledging survival of individual great tits—The effect of hatching date and fledging mass: Oikos, v. 3, no. 3, p. 481–488, accessed April 2020 at https://doi.org/10.1034/j.1600-0706.2002.11909.x.
Müller, Y.M.R., Rivero, L.B.D., Carvalho, M.C., Kobus, K., Farina, M., and Nazari, E.M., 2008, Behavioral impairments related to lead-induced developmental neurotoxicity in chicks: Archives of Toxicology, v. 82, p. 445–451, accessed June 2022 at https://doi.org/10.1007/s00204-007-0266-6.
Naef-Daenzer, B., Widmer, F., and Nuber, M., 2001, Differential post-fledging survival of great and coal tits in relation to their condition and fledging date: Journal of Animal Ecology, v. 70, no. 5, p. 730–738, accessed April 2020 at https://doi.org/10.1046/j.0021-8790.2001.00533.x.
Niethammer, K.R., Atkinson, R.D., Baskett, T.S., and Samson, F.B., 1985, Metals in riparian wildlife of the lead mining district of southeastern Missouri: Archives of Environmental Contamination and Toxicology, v. 14, no. 2, p. 213–223, accessed June 2019 at https://doi.org/10.1007/BF01055614.
North American Bluebird Society, 2016, Eastern/western bluebird nestbox (rev. January 2020): North American Bluebird Society, 3 p., accessed September 2019 at http://www.nabluebirdsociety.org/fact-sheets-plans/.
Nyholm, N.E.I., 1998, Influence of heavy metal exposure during different phases of the ontogeny on the development of pied flycatchers, Ficedula hypoleuca, in natural populations: Archives of Environmental Contamination and Toxicology, v. 35, no. 4, p. 632–637, accessed October 2019 at https://doi.org/10.1007/s002449900425.
Otis, D.L., Schulz, J.H., Miller, D., Mirarchi, R.E., and Baskett, T.S., 2008, Mourning Dove (Zenaida macroura) (ver. 2.0), in Poole, A.F., ed., The birds of North America: Ithaca, N.Y., Cornell Lab of Ornithology, accessed October 2019 at https://doi.org/10.2173/bna.117.
Pain, D.J., 1987, Lead poisoning in waterfowl—An investigation of sources and screening techniques: Oxford, United Kingdom, Doctor of Philosophy, University of Oxford, 335 p. [Also available at https://solo.bodleian.ox.ac.uk/permalink/f/89vilt/oxfaleph013844719.]
Pattee, O.H., 1984, Eggshell thickness and reproduction in American kestrels exposed to chronic dietary lead: Archives of Environmental Contamination and Toxicology, v. 13, no. 1, p. 29–34, accessed July 2019 at https://doi.org/10.1007/BF01055643.
Pavlowsky, R.T., Lecce, S.A., Owen, M.R., and Martin, D.J., 2017, Legacy sediment, lead, and zinc storage in channel and floodplain deposits of the Big River, Old Lead Belt Mining District, Missouri, USA: Geomorphology, v. 299, p. 54–75, accessed July 2019 at https://doi.org/10.1016/j.geomorph.2017.08.042.
Payne, R.B., 2006, Indigo bunting (Passerina cyanea) (ver. 2.0), in Poole, A.F., ed., The birds of North America: Ithaca, N.Y., Cornell Lab of Ornithology, accessed October 2019 at https://doi.org/10.2173/bna.4.
Peak, R.G., Thompson, F.R., III, and Shaffer, T.L., 2004, Factors affecting songbird nest survival in riparian forests in a Midwestern agricultural landscape: The Auk, v. 121, no. 3, p. 726–737, accessed June 2022 at https://academic.oup.com/auk/article/121/3/726/5561887.
Pinkowski, B.C., 1978, Feeding of nestling and fledgling eastern bluebirds: The Wilson Bulletin, v. 90, no. 1, p. 84–98, accessed October 2019 at https://sora.unm.edu/sites/default/files/journals/wilson/v090n01/p0084-p0098.pdf.
Pulliam, H.R., 1988, Sources, sinks, and population regulation: American Naturalist, v. 132, no. 5, p. 652–661, accessed August 2020 at https://www.jstor.org/stable/2461927.
Qiao, P., Lei, M., Yang, S., Yang, J., Guo, G., and Zhou, X., 2018, Comparing ordinary kriging and inverse distance weighting for soil as pollution in Beijing: Environmental Science and Pollution Research International, v. 25, p. 15597–15608, accessed June 2022 at https://doi.org/10.1007/s11356-018-1552-y.
R Core Team, 2019, R—A language and environment for statistical computing: R Foundation for Statistical Computing website, accessed April 2020 at https://www.R-project.org.
Rainio, M.J., Kanerva, M., Salminen, J.-P., Nikinmaa, M., and Eeva, T., 2013, Oxidative status in nestlings of three small passerine species exposed to metal pollution: Science of the Total Environment, v. 454–455, no. 1, p. 466–473, accessed October 2019 at https://doi.org/10.1016/j.scitotenv.2013.03.033.
Ralph, C.J., Geupel, G.R., Pyle, P., Martin, T.E., and DeSante, D.F., 1993, Handbook of field methods for monitoring landbirds: Albany, Calif., U.S. Department of Agriculture, Forest Service, Pacific Southwest Research Station, General Technical Report PSW–GTR–144, 41 p. [Also available at https://doi.org/10.2737/PSW-GTR-144.]
Remeš, V., Matysioková, B., and Cockburn, A., 2012, Long-term and large-scale analyses of nest predation patterns in Australian songbirds and a global comparison of nest predation rates: Journal of Avian Biology, v. 43, no. 5, p. 435–444, accessed April 2020 at https://doi.org/10.1111/j.1600-048X.2012.05599.x.
Ricklefs, R.E., 1969, An analysis of nesting mortality in birds: Smithsonian Contributions to Zoology, v. 9, p. 1–48, accessed April 2020 at https://doi.org/10.5479/si.00810282.9.
Ricklefs, R.E., 1984, The optimization of growth rate in altricial birds: Ecology, v. 65, no. 5, p. 1602–1616, accessed June 2022 at https://doi.org/10.2307/1939139.
Roach, M.C., Thompson, F.R., III, and Jones-Farrand, T., 2018, Songbird nest success is positively related to restoration of pine-oak savanna and woodland in the Ozark Highlands, Missouri, USA: The Condor, v. 120, no. 3, p. 543–556, accessed August 2020 at https://doi.org/10.1650/CONDOR-17-189.1.
Roux, K.E., and Marra, P.P., 2007, The presence and impact of environmental lead in passerine birds along an urban to rural land use gradient: Archives of Environmental Contamination and Toxicology, v. 53, no. 2, p. 261–268, accessed September 2019 at https://doi.org/10.1007/s00244-006-0174-4.
Sample, B.E., Hansen, J.A., Dailey, A., and Duncan, B., 2011, Assessment of risks to ground-feeding songbirds from lead in the Coeur d’Alene Basin, Idaho, USA: Integrated Environmental Assessment and Management, v. 7, no. 4, p. 596–611, accessed October 2019 at https://doi.org/10.1002/ieam.261.
Sample, B.E., Beyer, W.N., and Wentsel, R., 2019, Revisiting the avian Eco-SSL for lead—Recommendations for revision: Integrated Environmental Assessment and Management, v. 15, no. 5, p. 739–749, accessed August 2020 at https://doi.org/10.1002/ieam.4157.
Sauer, J.R., Niven, D.K., Hines, J.E., Ziolkowski, D.J., Jr., Pardieck, K.L., Fallon, J.E., and Link, W.A., 2017, The North American Breeding Bird Survey, results and analysis 1966–2015 (ver. 2.07.2017): Laurel, Md., U.S. Geological Survey digital data, accessed June 2022 at https://www.mbr-pwrc.usgs.gov/bbs/bbs.html.
Scheckel, K.G., Chaney, R.L., Basta, N.T., and Ryan, J.A., 2009, Advances in assessing bioavailability of metal(loid)s in contaminated soils, chap. 1 of Sparks, D.L., ed., Advances in Agronomy (v. 104): Amsterdam, The Netherlands, Academic Press, p. 1–52, accessed September 2021 at https://doi.org/10.1016/S0065-2113(09)04001-2.
Scheuhammer, A.M., 1987, The chronic toxicity of aluminium, cadmium, mercury, and lead in birds—A review: Environmental Pollution, v. 46, no. 4, p. 263–295, accessed October 2019 at https://doi.org/10.1016/0269-7491(87)90173-4.
Scheuhammer, A.M., and Norris, S.L., 1995, A review of the environmental impacts of lead shotshell ammunition and lead fishing weights in Canada: Canadian Wildlife Service, Occasional Paper 88, 56 p., accessed April 2020 at https://publications.gc.ca/collections/Collection/CW69-1-88E.pdf.
Schmitt, C.J., and McKee, M.J., 2016, Concentration trends for lead and calcium-normalized lead in fish fillets from the Big River—A mining-contaminated stream in southeastern Missouri USA: Bulletin of Environmental Contamination and Toxicology, v. 97, no. 5, p. 593–600, accessed April 2020 at https://doi.org/10.1007/s00128-016-1850-3.
Schmitt, C.J., Wildhaber, M.L., Hunn, J.B., Nash, T., Tieger, M.N., and Steadman, B.L., 1993, Biomonitoring of lead-contaminated Missouri streams with an assay for erythrocyte δ-aminolevulinic acid dehydratase activity in fish blood: Archives of Environmental Contamination and Toxicology, v. 25, no. 4, p. 464–475, accessed August 2020 at https://doi.org/10.1007/BF00214335.
Scinicariello, F., Murray, H.E., Moffett, D.B., Abadin, H.G., Sexton, M.J., and Fowler, B.A., 2007, Lead and δ-aminolevulinic acid dehydratase polymorphism—Where does it lead? A meta-analysis: Environmental Health Perspectives, v. 115, no. 1, p. 35–41, accessed June 2022 at https://doi.org/10.1289/ehp.9448.
Seeger, C.M., 2008, History of mining in the Southeast Missouri Lead District and description of mine processes, regulatory controls, environmental effects, and mine facilities in the Viburnum Trend subdistrict, in Kleeschulte, M.J., ed., Hydrologic investigations concerning lead mining issues in southeastern Missouri: U.S. Geological Survey Scientific Investigations Report 2008–5140, 238 p., accessed April 2020 at https://doi.org/10.3133/sir20085140.
Shaffer, T.L., 2004, A unified approach to analyzing nest success: The Auk, v. 121, no. 2, p. 526–540, accessed April 2020 at https://doi.org/10.2307/4090416.
Shaffer, T.L., and Thompson, F.R., III, 2007, Making meaningful estimates of nest survival with model-based methods: Studies in Avian Biology, v.34, p. 84–95, accessed August 2020 at https://sora.unm.edu/node/139712.
Snoeijs, T., Dauwe, T., Pinxten, R., Vandesande, F., and Eens, M., 2004, Heavy metal exposure affects the humoral immune response in a free-living small songbird, the great tit (Parus major): Archives of Environmental Contamination and Toxicology, v. 46, p. 399–404, accessed April 2020 at https://doi.org/10.1007/s00244-003-2195-6.
Snoeijs, T., Dauwe, T., Pinxten, R., Darras, V.M., Arckens, L., and Eens, M., 2005, The combined effect of lead exposure and high or low dietary calcium on health and immunocompetence in the zebra finch (Taeniopygia guttata): Environmental Pollution, v. 134, no. 1, p. 123–132, accessed April 2020 at https://doi.org/10.1016/j.envpol.2004.07.009.
Stansley, W., and Roscoe, D.E., 1996, The uptake and effects of lead in small mammals and frogs at a trap and skeet range: Archives of Environmental Contamination and Toxicology, v. 30, p. 220–226, accessed June 2022 at https://doi.org/10.1007/BF00215801.
Stone, C.L., Fox, M.R.S., Jones, A.L., and Mahaffey, K.R., 1977, δ-aminolevulinic acid dehydratase—A sensitive indicator of lead exposure in Japanese quail: Poultry Science, v. 56, no. 1, p. 174–181, accessed April 2020 at https://doi.org/10.3382/ps.0560174.
Thompson, F.R., III, 2007, Factors affecting nest predation on forest songbirds in North America: The Ibis, v. 149, no. 2, p. 98–109, accessed April 2020 at https://doi.org/10.1111/j.1474-919X.2007.00697.x.
Trust, K.A., Miller, M.W., Ringelman, J.K., and Orme, I.M., 1990, Effects of ingested lead on antibody production in mallards (Anas platyrhynchos): Journal of Wildlife Diseases, v. 26, no. 3, p. 316–322, accessed April 2020 at https://doi.org/10.7589/0090-3558-26.3.316.
Tsipoura, N., Burger, J., Newhouse, M., Jeitner, C., Gochfeld, M., and Mizrahi, D., 2011, Lead, mercury, cadmium, chromium, and arsenic levels in eggs, feathers, and tissues of Canada geese of the New Jersey Meadowlands: Environmental Research, v. 111, no. 6, p. 775–784, accessed April 2020 at https://doi.org/10.1016/j.envres.2011.05.013.
U.S. Environmental Protection Agency [EPA], 1996, Acid digestion of sediments, sludges, and soils [method 3050B, rev. 2]: U.S. Environmental Protection Agency, accessed April 2020 at https://www.epa.gov/esam/epa-method-3050b-acid-digestion-sediments-sludges-and-soils.
U.S. Environmental Protection Agency [EPA], 1997, Ecological risk assessment guidance for superfund—Process for designing and conducting ecological risk assessments: Edison, N.J., U.S. Environmental Protection Agency, Interim Final Paper, EPA 540–R–97-006, 239 p. [Also available at https://semspub.epa.gov/work/HQ/157941.pdf.]
U.S. Environmental Protection Agency [EPA], 2007, Field portable x-ray fluorescence spectrometry for the determination of elemental concentrations in soil and sediment [method 6200, rev. 0]: U.S. Environmental Protection Agency, 32 p., accessed September 2021 at https://www.epa.gov/sites/default/files/2015-12/documents/6200.pdf.
U.S. Environmental Protection Agency [EPA], 2014, Inductively coupled plasma-mass spectrometry [method 6020B (SW–846), rev. 2]: U.S. Environmental Protection Agency, 33 p., accessed April 2020 at https://www.epa.gov/esam/epa-method-6020b-sw-846-inductively-coupled-plasma-mass-spectrometry.
U.S. Fish and Wildlife Service [USFWS] and Missouri Department of Natural Resources, 2014, Preassessment screen and determination, Madison County Mines Site, Madison County, MO: U.S. Fish and Wildlife Service. [Also available at https://www.cerc.usgs.gov/orda_docs/DocHandler.ashx?task=get&ID=1391.]
Vallverdú-Coll, N., Mateo, R., Mougeot, F., and Ortiz-Santaliestra, M.E., 2019, Immunotoxic effects of lead on birds: Science of the Total Environment, v. 689, p. 505–515, accessed August 2020 at https://doi.org/10.1016/j.scitotenv.2019.06.251.
Vallverdú-Coll, N., Mougeot, F., Ortiz-Santaliestra, M.E., Rodriguez-Estival, J., López-Antia, A., and Mateo, R., 2016, Lead exposure reduces carotenoid-based coloration and constitutive immunity in wild mallards: Environmental Toxicology and Chemistry, v. 35, no. 6, p. 1516–1525, accessed April 2020 at https://doi.org/10.1002/etc.3301.
Visser, M.E., and Verboven, N., 1999, Long-term fitness effects of fledging date in great tits: Oikos, v. 85, no. 3, p. 445–450, accessed April 2020 at https://doi.org/10.2307/3546694.
Vyas, N.B., Spann, J.W., and Heinz, G.H., 2001, Lead shot toxicity to passerines: Environmental Pollution, v. 111, no. 1, p. 135–138, accessed June 2022 at https://doi.org/10.1016/S0269-7491(99)00333-4.
Vyas, N.B., Spann, J.W., Heinz, G.H., Beyer, W.N., Jaquette, J.A., and Mengelkoch, J.M., 2000, Lead poisoning of passerines at a trap and skeet range: Environmental Pollution, v. 107, no. 1, p. 159–166, accessed April 2020 at https://doi.org/10.1016/S0269-7491(99)00112-8.
Weber, J.S., Goyne, K.W., Luxton, T.P., and Thompson, A.L., 2015, Phosphate treatment of lead-contaminated soil—Effects on water quality, plant uptake, and lead speciation: Journal of Environmental Quality, v. 44, no. 4, p. 1127–1136, accessed September 2019 at https://doi.org/10.2134/jeq2014.10.0447.
Wheelwright, N.T., 1986, The diet of American robins—An analysis of U.S. biological survey records: The Auk, v. 103, p. 710–725, accessed October 2019 at https://sora.unm.edu/sites/default/files/journals/auk/v103n04/p0710-p0725.pdf.
Willner, G.R., Gates, J.E., and Devlin, W.J., 1983, Nest box use by cavity-nesting birds: American Midland Naturalist, v. 109, no. 1, p. 194–201, accessed October 2019 at https://doi.org/10.2307/2425530.
Woodward, A.A., Fink, A.D., and Thompson, F.R., III, 2001, Edge effects and ecological traps—Effects on shrubland birds in Missouri: The Journal of Wildlife Management, v. 65, no. 4, p. 668–675, accessed June 2022 at https://www.jstor.org/stable/3803018.
Yan, K., Dong, Z., Wijayawardena, M.A.A., Liu, Y., Naidu, R., and Semple, K., 2017, Measurement of soil lead bioavailability and influence of soil types and properties—A review: Chemosphere, v. 184, p. 27–42, accessed September 2021 at https://doi.org/10.1016/j.chemosphere.2017.05.143.
Yang, J., Mosby, D.E., Casteel, S.W., and Blanchar, R.W., 2002, In vitro lead bioaccessibility and phosphate leaching as affected by surface application of phosphoric acid in lead-contaminated soil: Archives of Environmental Contamination and Toxicology, v. 43, no. 4, p. 399–405, accessed August 2020 at https://doi.org/10.1007/s00244-002-1197-0.
Young, H., 1956, Territorial activities of the American robin Turdus migratorius: The Ibis, v. 98, no. 3, p. 448–452, accessed September 2019 at https://doi.org/10.1111/j.1474-919X.1956.tb01429.x.
Appendix 1. Photographic Log of Nesting Sites and Habitats in the Southeast Missouri Lead Mining District
Reference Sites
Hawn State Park (Ste. Genevieve County, Mo.)

A recently burned field of Festuca L. spp. (fescue) early in the field season, April 2018, at Hawn State Park. Land managers were converting the habitat to pine savanna at the time of this photograph. Photograph by Kathy Hixson, Southeast Missouri State University.

An old field bordered by Pinus L. spp. (pine) and Quercus L. spp. (oak) woodlands at Hawn State Park. Photograph by Kathy Hixson, Southeast Missouri State University.

Quercus L. spp. (oak) and Carya Nutt. spp. (hickory) forest in the middle of the 2016 field season, July 2016, at Hawn State Park. A Sialia sialis (Linnaeus, 1758) (eastern bluebird)/S. mexicana (Swainson, 1832) (western bluebird) nest box is visible at the right in the middle-distance. Photograph by Kathy Hixson, Southeast Missouri State University.

An old field bordered by Quercus L. spp. (oak) and Carya Nutt. spp. (hickory) forest at Hawn State Park. Photograph by Kathy Hixson, Southeast Missouri State University.

A Sialia sialis (Linnaeus, 1758) (eastern bluebird)/S. mexicana (Swainson, 1832) (western bluebird) nest box, with predator guard, deployed at Hawn State Park for the 2016 field season. The nest box was in Pinus L. spp. (pine) savanna next to old field habitat. Photograph by Kathy Hixson, Southeast Missouri State University.

Pinus L. spp. (pine) savanna next to an old field early in the field season, March 2016, at Hawn State Park. Photograph by Kathy Hixson, Southeast Missouri State University.

A researcher uses an inspection mirror to monitor a Cardinalis cardinalis (Linnaeus, 1758) (northern cardinal) nest at Hawn State Park. Photograph by Kathy Hixson, Southeast Missouri State University.

Passerina cyanea (Linnaeus, 1766) (indigo bunting) nest, with eggs visible via inspection mirror, at Hawn State Park. Photograph by Melissa Roach, University of Missouri–Columbia.

A researcher uses an inspection mirror to investigate a Passerina cyanea (Linnaeus, 1766) (indigo bunting) nest at Hawn State Park. Photograph by Melissa Roach, University of Missouri–Columbia.

Spizella pusilla (A. Wilson, 1810) (field sparrow) eggs in a nest in an old field at Hawn State Park. Photograph by Melissa Roach, University of Missouri–Columbia.

Pipilo erythrophthalmus (Linnaeus, 1758) (eastern towhee) eggs in a nest at Hawn State Park. Photograph by Melissa Roach, University of Missouri–Columbia.
Johnson’s Shut-ins State Park (Reynolds County, Mo.)

Sialia sialis (Linnaeus, 1758) (eastern bluebird)/S. mexicana (Swainson, 1832) (western bluebird) nest box deployed in an old field at Johnson’s Shut-ins State Park. Photograph by Kathy Hixson, Southeast Missouri State University.

Nest box deployed in a mowed field at Johnson’s Shut-ins State Park. Photograph by Kathy Hixson, Southeast Missouri State University.

Field and forest habitats early in the field season, April 2016, at Johnson’s Shut-ins State Park. Photograph by Kathy Hixson, Southeast Missouri State University.

Old field and savanna in April 2016 at Johnson’s Shut-ins State Park. Photograph by Kathy Hixson, Southeast Missouri State University.

Old field with savanna and woodland in the background taken mid-season, July 2016, at Johnson’s Shut-ins State Park. Photograph by Kathy Hixson, Southeast Missouri State University.
Contaminated Sites
Anschutz Mine (Madison County, Mo.)
Passerina cyanea (Linnaeus, 1766) (indigo bunting) chicks in a nest at Anschutz Mine. Photograph by Melissa Roach, University of Missouri–Columbia.

A researcher searches for a Passerina cyanea (Linnaeus, 1766) (indigo bunting) nest in forest habitat at Anschutz Mine. Photograph by Kathy Hixson, Southeast Missouri State University.

A Spizella pusilla (A. Wilson, 1810) (field sparrow) nest with eggs in a Juniperus virginiana L. (eastern redcedar) tree at Anschutz Mine. Photograph by Melissa Roach, University of Missouri–Columbia.

A Spizella pusilla (A. Wilson, 1810) (field sparrow) nest and eggs in field habitat at Anschutz Mine. Photograph by Melissa Roach, University of Missouri–Columbia.

Juniperus virginiana L. (eastern redcedar) and scrub-shrub vegetation on capped mine tailings taken mid-field season, July 2017, at Anschutz Mine. Tailings were capped with uncontaminated soil as part of cleanup activities by the U.S. Environmental Protection Agency. Photograph by Kathy Hixson, Southeast Missouri State University.

Mixed deciduous forest habitat next to a road and capped tailings at Anschutz Mine. Photograph by Kathy Hixson, Southeast Missouri State University.

Mixed Pinus L. spp. (pine) and deciduous woodlands next to a road at Anschutz Mine Photograph by Kathy Hixson, Southeast Missouri State University.

Mixed Pinus L. spp. (pine) and deciduous forest next to mine tailings at Anschutz Mine. Photograph by Kathy Hixson, Southeast Missouri State University.

Mixed Pinus L. spp. (pine), Juniperus virginiana L. (eastern redcedar), and scrub-shrub vegetation at Anschutz Mine. Photograph by Kathy Hixson, Southeast Missouri State University.

Mixed deciduous forest next to recent grading and capping remediation work in the 2016 field season at Anschutz Mine. Photograph by Kathy Hixson, Southeast Missouri State University.

Researchers pursue a Cardinalis cardinalis (Linnaeus, 1758) (northern cardinal) in scrub-shrub vegetation at Anschutz Mine. Photograph by Kathy Hixson, Southeast Missouri State University.
Big River floodplain (DeSoto, Mo.)

Bottomland forest and field at a public day-use area in Washington State Park in the Big River floodplain. Photograph by Kathy Hixson, Southeast Missouri State University.

A nest box installed in bottomland forest and field habitat mid-field season, April 2016, at Washington State Park in the Big River floodplain. Photograph by Kathy Hixson, Southeast Missouri State University.

Bottomland forest early in the field season, April 2016, at Washington State Park in the Big River floodplain. Photograph by Kathy Hixson, Southeast Missouri State University.

Mowed field and riparian forest early in the field season, April 2016, on a private land tract in the Big River floodplain. Photograph by Kathy Hixson, Southeast Missouri State University.

Mowed field and forest early in the field season, March 2017, on a private land tract in the Big River floodplain. Photograph by Kathy Hixson, Southeast Missouri State University.

Riparian forest early in the field season on a private land tract in the Big River floodplain. Photograph by Kathy Hixson, Southeast Missouri State University.

Mowed field and riparian forest on a private land tract in the Big River floodplain. Photograph by Kathy Hixson, Southeast Missouri State University.

A Passerina cyanea (Linnaeus, 1766) (indigo bunting) nest at Washington State Park in the Big River floodplain. Photograph by Melissa Roach, University of Missouri–Columbia.

A Passerina cyanea (Linnaeus, 1766) (indigo bunting) nest (circled) at Washington State Park in the Big River floodplain. Photograph by Melissa Roach, University of Missouri–Columbia.

Fuchsia flagging indicating the location of a Passerina cyanea (Linnaeus, 1766) (indigo bunting) nest in floodplain forest at Washington State Park in the Big River floodplain. Each nest was designated with a unique identification code and marked with weather-resistant flagging placed greater than or equal to 5 meters from the nest. Photograph by Melissa Roach, University of Missouri–Columbia.

Floodplain forest mid-season, May 2018, at Washington State Park in the Big River floodplain. Photograph by Melissa Roach, University of Missouri–Columbia.

Riparian forest surrounding the Big River at Washington State Park in the Big River floodplain. Photograph by Kathy Hixson, Southeast Missouri State University.

A mixture of mowed field, old field, and riparian forest at the Washington State Park Big River public day-use area in the Big River floodplain. Photograph by Kathy Hixson, Southeast Missouri State University.
Magmont Vent (Iron County, Mo.)

A utility cut through deciduous woodland habitat mid-field season, July 2016, at Magmont Vent. Photograph by Kathy Hixson, Southeast Missouri State University.

A bird captured in a mist net that had been deployed along a paved road through woodland habitat at Magmont Vent. Photograph by Kathy Hixson, Southeast Missouri State University.

A Passerina cyanea (Linnaeus, 1766) (indigo bunting) nest in a Quercus L. sp. (oak) tree at Magmont Vent. Photograph by Melissa Roach, University of Missouri–Columbia.

A Spizella pusilla (A. Wilson, 1810) (field sparrow) nest in a Juniperus virginiana L. (eastern redcedar) tree at Magmont Vent. The nest is in the white circle. Photograph by Melissa Roach, University of Missouri–Columbia.

A Pipilo erythrophthalmus (Linnaeus, 1758) (eastern towhee) nest (circled) in a Juniperus virginiana L. (eastern redcedar) tree at Magmont Vent. Photograph by Melissa Roach, University of Missouri–Columbia.

A Pipilo erythrophthalmus (Linnaeus, 1758) (eastern towhee) (circled) in the understory at Magmont Vent. Photograph by Melissa Roach, University of Missouri–Columbia.

Utility line through deciduous forest mid-season at Magmont Vent. Photograph by Kathy Hixson, Southeast Missouri State University.
A Sialia sialis (Linnaeus, 1758) (eastern bluebird)/S. mexicana (Swainson, 1832) (western bluebird) nest box, with predator guard, deployed in Pinus L. spp. (pine), Quercus L. spp. (oak), and scrub-shrub vegetation at Magmont Vent. Photograph by Kathy Hixson, Southeast Missouri State University.

A Cardinalis cardinalis (Linnaeus, 1758) (northern cardinal) nest (circled) in understory at Magmont Vent. Photograph by Melissa Roach, University of Missouri–Columbia.

A nest box in a clearing at Magmont Vent. The vent for the Casteel Mine (Bixby, Mo.) is visible in the cage structure in the background. Photograph by Kathy Hixson, Southeast Missouri State University.

A Sialia sialis (Linnaeus, 1758) (eastern bluebird)/S. mexicana (Swainson, 1832) (western bluebird) nest box deployed in a utility cut through deciduous woodland mid-season, July 2016, at Magmont Vent. Photograph by Kathy Hixson, Southeast Missouri State University.
Appendix 2. Quality Control of Analyses in the Southeast Missouri Lead Mining DistrictByGiven (First) Names: DanielleCleveland, Given (First) Names: David E.Mosby, Given (First) Names: Barnett A.Rattner, and Given (First) Names: Natalie K.Karouna-Renier
Introduction
This appendix describes the quality control (QC) measures and results, which were not described in the main text for the purpose of conciseness, that were obtained during the quantification of δ-aminolevulinic acid dehydratase (δALAD) activity; oxidative stress markers; DNA damage; and lead in soil, invertebrates, and avian tissues and used to evaluate the accuracy and precision of the results. In addition, we provide results of confirmatory analyses for lead in soil samples. Metadata and digital datasets for data generated by the U.S. Geological Survey are available, per data management policy, in a U.S. Geological Survey data release (Cleveland and others, 2023).
Quality Control for Portable X-Ray Fluorescence Spectrometry
Several different portable X-ray fluorescence spectrometry (pXRF) units were used over the 3-year study (2016–19). All offsite analyses were performed using a Thermo Scientific Niton XL3t 600 XRF Analyzer (Thermo Scientific, Billerica, Massaschusetts). Onsite analyses were performed using the Niton XL3t 600, a Thermo Scientific Niton XL2GOLDD (Thermo Scientific, Model XL2980, Billerica, Mass.), an Innov-X Delta XRF Analyzer Standard DS-4000 Model (Innov-X Technologies, Vancouver, British Columbia, Canada), or an Olympus Delta Environmental DS–4000 (Olympus Scientific Solutions America, Center Valley, Pennsylvania). The QC analyses for pXRF consisted of using eight certified reference materials (CRMs) from the National Institute of Standards and Technology (Gaithersburg, Maryland) to verify instrument performance. The CRMs were soil or sediment matrices that had lead concentrations ranging from 27 to 5,532 milligrams of lead per kilogram of CRM (mg/kg). This concentration range represented the approximate range of lead concentrations that was expected at the study sites (reference and contaminated). Note that lead in the CRMs was certified in units of milligrams per kilogram on a dry-weight (dw) basis; measurement units for pXRF are typically given as parts per million. For quantification of lead in soil, and for comparison to pXRF results to the certified lead concentrations in the CRMs, 1 part per million was considered to be equivalent to 1 mg/kg dw. The relative percent differences (RPDs) of duplicate pXRF measurements of lead concentrations ranged from 1.1 to 25 percent. Silicon dioxide was used as a negative control material; all silicon dioxide samples had lead concentrations below the instrument detection limits provided by the pXRF manufacturers (table 2.1).
Table 2.1.
Estimated limits of detection, limits of quantification, and reporting limits.[All data except those for soil are summarized from Cleveland and others (2023). pXRF, portable X-ray fluorescence spectrometry; IDL, instrument detection limit; ppm, part per million; ICP–MS, inductively coupled plasma-mass spectrometry; LOQ, estimated limit of quantification; mg/kg dw, milligrams of analyte per kilogram of sample on a dry-weight basis; δALAD, δ-aminolevulinic acid dehydratase; LOD, estimated limit of detection; TSH, total sulfhydryl; μM, micromolar or micromoles of analyte per liter; GSH, reduced glutathione; <, less than; tGSH, total glutathione; TBARS, thiobarbituric acid reactive substances; RL, reporting limit; DNA, deoxyribonucleic acid; 8-OH-dG, 8-hydroxy-2′-deoxyguanosine; ng/mL, nanogram of analyte per milliliter of sample]
Quality Control for Inductively Coupled Plasma-Mass Spectrometry
Lead in Invertebrates
QC measures incorporated into the analysis of lead in invertebrates by inductively coupled plasma-mass spectrometry (ICP–MS; PerkinElmer DRC-e or NexION 2000P, Shelton, Connecticut) were within acceptable limits for the method. Recoveries of lead in the calibration standards were 90 to 110 percent; and the internal standard recoveries were 60 to 120 percent throughout the analyses. The second-source initial calibration verification standard (ICVS) and continuing calibration verification standards (CCVSs) and blanks were analyzed every 10 samples; recoveries of lead from the ICVS and CCVSs were 90 to 110 percent. Lead recoveries from the laboratory control solutions were 90.3 to 100.8 percent. Method and analytical spike recoveries ranged from 97.6 to 101 percent, and analytical dilutions had less than (<) 2 RPD. A method triplicate for worm tissue had a relative standard deviation of 28.3 percent, likely because of sample heterogeneity due to the presence of soil particulates in the tissue sample. Analytical duplicate RPDs were 1.3 to 1.8 percent. Recoveries of lead from CRMs consisting of invertebrate tissues (for example, mussels and oysters) ranged from 87 to 100 percent of the certified range. The mean blank equivalent lead concentration in the procedural blanks was 0.03 mg/kg dw, and the estimated limits of quantification (LOQ) for lead in the tissues were 0.1 to 0.2 mg/kg dw (table 2.1). Results were censored at the LOQ level.
Lead in Blood, Kidneys, and Livers
QC measures incorporated into the ICP–MS analysis of lead in avian blood, liver, and kidney tissues were within the acceptable limits for the method. Recoveries of lead in the calibration standards were 90 to 110 percent; and the internal standard recoveries were 60 to 120 percent. As with invertebrates, second source ICVS and CCVSs and blanks were analyzed every 10 samples; recoveries of lead from the ICVS and CCVSs were 90 to 110 percent. Lead recoveries from the laboratory control solutions were 85.8 to 117 percent. Method and analytical spike recoveries ranged from 89.8 to 108 percent, and analytical dilutions had <5 RPD. Method triplicates had relative standard deviations of 0.8 to 4.2 percent. Analytical duplicate RPDs were 0 to 4.8 percent. Recoveries of lead from CRMs having similar matrices (for example, bovine liver and whole blood) ranged from 80.8 to 114 percent of the certified range, except for 1 CRM used during liver and kidney analysis, which had a lead recovery of 162 percent for 1 of 3 analyses, and 1 whole blood CRM, which had lead recoveries of 66.2 to 79.4 percent lead for 3 of 11 analyses (that is, 8 of 11 were in the 80.8 to 114 percent recovery range for this material). However, for kidney and liver analyses, the weight of CRM used (40 milligrams) was well below the weight (250 milligrams) at which the material was tested for homogeneity; this discrepancy likely affected the lead recovery. Similarly, the weight of blood CRM (20 milligrams) used for digestions was low in comparison to the recommended weight (5 milliliters of reconstituted material). The mean blank equivalent concentration for lead in the procedural blanks was 0.01 mg/kg dw. The estimated LOQs for lead in the livers and kidneys were 0.03 to 0.2 mg/kg dw (table 2.1); the estimated LOQs for lead in blood were 0.02 to 0.1 mg/kg dw. Results were censored at the LOQ level.
Quality Control for δ-Aminolevulinic Acid Dehydratase Assays
Preliminary δALAD assays using commercially available heparinized laboratory Mus musculus (Linnaeus, 1758) (mouse) and Gallus gallus (Linnaeus, 1758) (chicken) whole blood (BioIVT, Westbury, New York) were performed to verify assay linearity over incubation time and sample volume, characterize the precision of quadruplicate analyses, and to estimate the limit of detection (LOD). Incubation of varying volumes of mouse and chicken blood (12, 25, 37 and 50 microliters) for 60 minutes yielded comparable blank-corrected absorbances when normalized on a unit volume basis (mean plus or minus [±] standard deviation [SD]; mouse=0.198±0.017; chicken=0.157±0.022). A different pool of chicken blood was analyzed on different days (August 9 and 13, 2018); both pools yielded comparable mean blank-corrected absorbances when normalized on a unit volume basis (mean absorbance ± SD of 0.072±0.012 and 0.082±0.014). Incubation of 25 microliters samples of mouse and chicken blood for varying durations (30, 60, and 90 minutes) yielded comparable mean blank-corrected absorbances (mouse=0.191±0.014; chicken=0.161±0.005). Chicken blood assays having varying incubation times were repeated on two additional days and yielded comparable mean blank-corrected absorbances of 0.073±0.003 and 0.086±0.014. Intra-assay precision using quadruplicate determinations of mouse and chicken blood (25 microliters) yielded mean blank-corrected absorbances of 0.194±0.009 and 0.164±0.005, respectively. Tests for intra-assay precision were repeated for chicken blood on two additional days and yielded mean blank-corrected absorbances of 0.072±0.005 and 0.081±0.008. An estimated LOD for the δALAD activity assay (table 2.1) was determined based on analysis of 10 chicken sample replicates without the addition of substrate. Replicate analyses yielded a mean absorbance (±2 SDs) of 0.032; this corresponds to 13.99 units of activity assuming a hematocrit of 40.
A total of 52 unique field samples were assayed in duplicate in three batches (consisting of 6, 18, and 28 samples, respectively); chicken blood was assayed multiple times in each batch. The mean coefficient of variation (CV; mean CV±SD) for duplicate determinations was 5.62±3.67 percent. For chicken blood samples, the average intra-batch CV for blank-corrected absorbance ranged from 1.93 to 8.64 percent, and the inter-batch CV for blank-corrected absorbance was 5.01±3.39 percent. We note that one Turdus migratorius (Linnaeus, 1766) (American robin) sample from Cape Girardeau was missing a microhematocrit value and could not be analyzed for δALAD; a Cardinalis cardinalis (Linnaeus, 1758) (northern cardinal) blood sample from the Big River floodplain site was clotted and could also not be analyzed. One Molothrus ater (Boddaert, 1783) (brown-headed cowbird) sample from Cape Girardeau was clotted but could still be pipetted; however, the δALAD results for that sample were excluded from statistical treatments.
Quality Control for Oxidative Stress Indicator Analyses
Interassay CVs for the two reference samples were 9.29 and 1.47 percent for total sulfhydryl, 6.06 and 9.39 percent for reduced glutathione, and 5.38 and 8.44 percent for thiobarbituric acid reactive substances. The LOD (3× the SD of 10 wells containing assay diluent) was 1.28 micromoles per liter (µM) for total sulfhydryl, <0.001 µM for reduced glutathione, 0.53 µM for total glutathione, and 0.03 µM for thiobarbituric acid reactive substances (table 2.1). The reporting limits, which were determined from the lowest calibration standard for which replicates had CVs<15 percent, were 6.88 µM for total sulfhydryl, 0.78 µM for reduced glutathione and total glutathione, and 0.094 µM for thiobarbituric acid reactive substances (table 2.1).
Quality Control for Deoxyribonucleic Acid Damage Indicator Analyses
Standard calibration curves (0.125 to 4 nanograms per milliliter; coefficient of determination [R2] greater than 0.999), which were used to calibrate the microplate reader response, were fit using a four-parameter model (MARS Data Analysis Software 2.10 R3; BMG LABTECH, Inc., Cary, North Carolina.). The LOD was <0.001 nanogram per milliliter and the reporting limit was 0.25 nanogram per milliliter (table 2.1). Interassay variability (that is, percent CV) of the two reference samples were 8.3 and 1.1 percent.
Confirmation of Results for Lead in Soil
Onsite pXRF analyses of soils have been widely used to characterize surficial contamination at large study sites in a cost-effective manner (for example, U.S. Environmental Protection Agency [EPA] method 6200; EPA, 2007). A pXRF unit can be used to collect large numbers of data points relatively rapidly, with little-to-no sample preparation and limited operator expertise. Further, pXRF analyses are nondestructive, which permits re-analysis of the sample later or by another methodology. However, there are limitations to pXRF analyses, including matrix effects (for example, sample particle size and heterogeneity), moisture and spectral interferences, and reduced sensitivity relative to more sophisticated, laboratory-based methods, including ICP–MS.
We generally used onsite pXRF to measure the lead concentrations in soil along transects at our study sites to characterize the degree of contamination to which songbirds might be exposed while foraging in their breeding territories in the Southeast Missouri Lead Mining District. However, the pXRF technique is sensitive to sample moisture content (Sahraoui and Hachicha, 2017); pXRF manufacturer guidance suggests that concentration results can be biased low when soil moistures exceed about 20 percent. Therefore, soil samples for offsite pXRF analyses were collected if the area of soil was too wet for onsite analyses to be reliable (in other words, visible water or puddles were within 1 m of the location). Soil samples for offsite analyses consisted of five to six equal aliquots of soil collected at a depth of 5 to 10 centimeters from a 100-square-meter area (totaling about 1 kilogram of soil). We note that the soil conditions were saturated at many of the contaminated sites during 2018; therefore, only offsite samples were collected. Occasional low-lying areas in the contaminated sites during the 2016 and 2017 breeding seasons were also affected by wet soil conditions and required offsite pXRF methods. Soil samples collected for offsite pXRF analyses were air dried and sieved (<2 millimeters) before analyses.
In addition, we used duplicate analyses to confirm a subset of the onsite pXRF results. Duplicate soil samples were analyzed onsite for lead concentrations and then collected and returned to the laboratory for offsite lead analysis by pXRF or ICP–MS after microwave-assisted acid digestion. Duplicate samples were collected at approximate rates of 33 percent for offsite pXRF analyses (that is, n=1 in about 3 soil samples having onsite quantification of lead were also collected for offsite duplicate analyses) and 10 percent for ICP–MS analyses (that is, n=1 in about 10 soil samples collected for duplicate offsite analyses were split and for ICP–MS analyses). As before, the duplicate samples were air dried and sieved (<2 millimeter) before offsite pXRF analyses; samples that were to be analyzed by ICP–MS were further dried by lyophilization and manually homogenized before acid digestion. A comparison of the lead concentrations measured by each method is given in table 2.2; and graphical comparisons of the results are given in figure 2.1. Note that in figure 2.1, the dotted line indicates a one-to-one (1:1) relationship between the two compared techniques; in other words, data pairs between the two techniques that are closer to the 1:1 line are in better agreement than result pairs further from the line.
Table 2.2.
Concentrations of lead in soil samples, quantified by two or more analytical methods.[A concentration of 1 part per million (ppm) by portable X-ray fluorescence spectrometry (pXRF) is approximately equivalent to 1 milligram per kilogram (mg/kg) dry weight (dw) for analyses by other quantification methods, including inductively coupled plasma-mass spectrometry (ICP–MS). NM, not measured by this method]
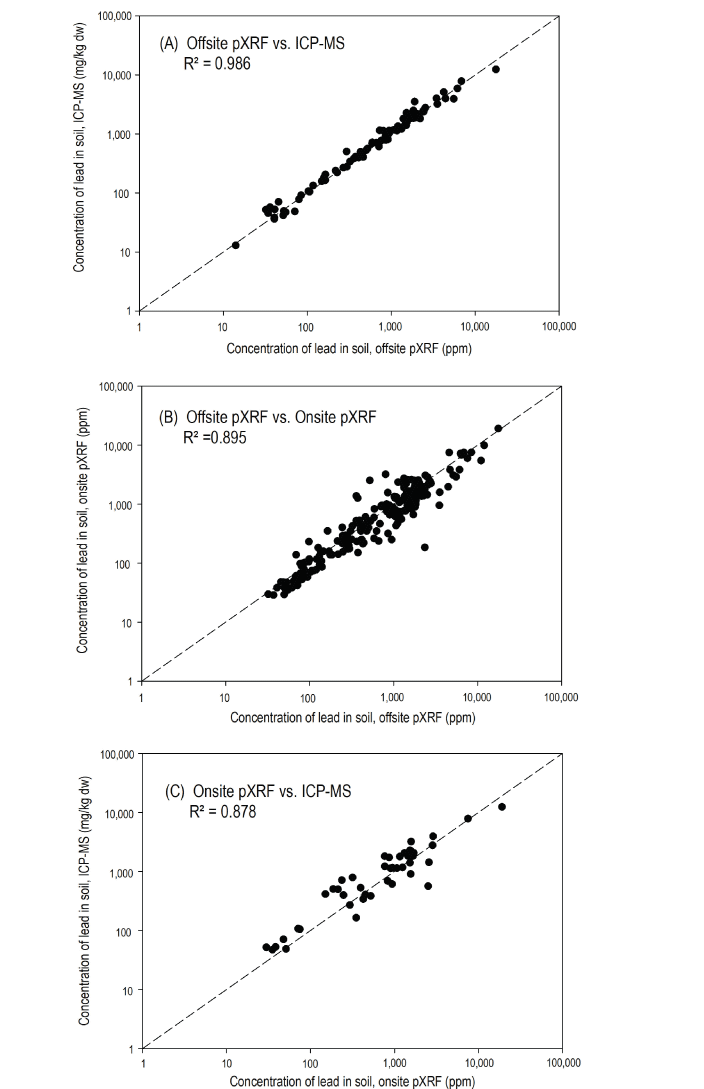
Comparisons of concentrations of lead measured in duplicate soil samples. A, offsite portable X-ray fluorescence spectrometry (pXRF) compared to inductively coupled plasma-mass spectrometry (ICP–MS). B, offsite pXRF compared to onsite pXRF. C, onsite pXRF compared to ICP–MS.
It is important to note that, as with all analytical techniques, there are potential limitations to both the pXRF (onsite and offsite) and ICP–MS methods, which may reduce comparability of the results. For example, pXRF is generally considered to measure concentrations of metals on a “total” or “near total” basis (under optimal conditions), whereas our ICP–MS method used a sample digestion method similar to EPA method 3050B (EPA, 1996), which accesses the “total recoverable” fraction of metal to assess concentrations that might become available to the environment or biota. A “total recoverable” approach generally does not access the fraction of metal associated with silica, whereas a “total” method would access the silica-bound metals. In this way, a pXRF result might be expected to be biased high against an ICP–MS result for the same sample if the sample contains significant fractions of lead bound in silicates.
Sample moisture can also affect comparisons of results obtained by different methods. It is generally recommended that samples intended for pXRF analyses have moisture <20 percent; ICP–MS measurements are generally performed on samples that have been lyophilized and are fully dried. However, moisture levels greater than 20 percent are not always readily apparent in onsite soils, and drying can significantly slow site characterization and increase costs. An onsite sample having more moisture than its offsite dried counterpart might be reasonably expected to have a lower perceived lead concentration compared to dried samples because of “dilution” of the lead by interstitial water in the soil; this error can be compounded because the calibration of the pXRF detector is verified using dry reference materials.
It should also be noted that pXRF is a surface technique that interrogates a sample to a fixed depth; and as such, accuracy is affected by numerous sample characteristics (Ravansari and others, 2020). Factors include the density and geometry of the sample, particle size, the heterogeneity of elements both within the sample as a whole and among the different particle sizes, and complex matrix interferences (for example, attenuation of fluorescence via absorption or scattering; enhancement of fluorescence). For example, in an onsite experiment where pXRF readings are taken from an undisturbed substrate, smaller-sized dust particulates, which might be expected to have greater concentrations of sorbed metals than their larger-sized counterparts (for example, Stone and Droppo, 1996), might be present in greater numbers at the surface. In this way, the measured concentration of metals in the soil could be elevated. In contrast, in an offsite experiment where the sample has been bagged and includes some portion of below-ground sediment, smaller-sized particles might be expected to fall to the bottom of the sample container, leaving the larger-sized particles more in-view of the pXRF detector. The ICP–MS procedure can also be affected by particle size; although care is generally taken to aliquot a representative part of the sample, the accuracy of the results is incumbent upon the analysts and their cognizance of the importance of sample homogeneity. The recommendation for pXRF and ICP–MS analyses is the use of highly homogeneous samples having fine-grained particles with limited particle size distribution; however, this is not always realistic with environmental samples. Nonetheless, we believe we captured a reasonable portrait of the lead concentrations to which songbirds were exposed while foraging.
Quality Control for Quantification of Lead in Soils by ICP–MS
QC measures incorporated into the ICP–MS analysis of lead in confirmatory soil samples were within the acceptable limits for the method. Recoveries of lead in the calibration standards were 90 to 110 percent; and the internal standard recoveries were 60 to 120 percent throughout the analyses. Second source ICVS and CCVSs and blanks were analyzed every 10 samples; recoveries of lead from the ICVS and CCVSs were 90 to 110 percent. Lead recoveries from the laboratory control solutions were 90.0 to 102.2 percent. Method and analytical spike recoveries ranged from 94.2 to 109.8 percent, and analytical dilutions had <3 RPDs. Method triplicates had relative standard deviations of 1.0 to 9.4 percent, and analytical duplicates had RPDs of 0.6 to 3.9 percent. Recoveries of lead from CRMs (soils and sediments) ranged from 87.3 to 100 percent of the certified range. The mean blank equivalent concentrations for lead in the procedural blanks was 0.02 mg/kg dw, and the estimated LOQ for lead in the tissues were 0.1 to 0.2 mg/kg dw (table 2.1). Results were censored at the LOQ level.
Comparison of Lead Concentrations Measured in Soils by Offsite pXRF and ICP–MS
There were 89 soil samples having results for lead obtained by both offsite pXRF and ICP–MS quantification methods (table 2.2). The RPDs between the two results ranged from 0 to 60.0 percent, the mean RPD (± standard error) was 13.9±1.4 percent, and the median RPD was 9.3 percent. We compared the results for the two analytical methods using Spearman’s rank correlation; the Spearman’s correlation coefficient was 0.989 (probability value was <0.001). We also modeled the data using linear regression of the results for the two different methodologies; the regression had a R2 of 0.986 at a 95-percent confidence level (fig. 2.1A). Differences between the lead concentrations measured in soil by offsite pXRF and ICP–MS were not particularly surprising, given the sensitivity of pXRF to moisture, particle size, and sample heterogeneity, as well as the differences in quantification strategy between the two methods (for example, “total lead” for pXRF versus “total recoverable lead” for ICP–MS). Overall, the ICP–MS results validated the performance of offsite pXRF for quantification of lead in soils.
Comparison of Lead Concentrations in Soil by Onsite pXRF and Offsite pXRF
There were 224 samples having onsite and offsite pXRF results for lead (table 2.2). RPDs between the two results ranged from 0 to 171 percent; the mean RPD (±SE) was 33.2±6.2 percent and the median RPD was 26.4 percent. Two samples had RPD=0 percent for onsite and offsite results. Linear regression of the results for the two different methodologies had R2=0.895 at a 95-percent confidence level (fig. 2.1B); and Spearman’s correlation coefficient was 0.903 (probability value was <0.001). Differences between the lead concentrations measured in soil by onsite and offsite pXRF were also not particularly surprising, given the sensitivity of pXRF to moisture and sample heterogeneity. The lead concentration measured on site in soils was less than the concentration of lead measured off site in duplicate samples for n=150 of 224 measurements (67 percent, table 2.1), which suggests that the presence of moisture reduced the apparent lead concentrations of onsite soils compared to offsite portions. Differences between the onsite and offsite measurement results were not limited to samples having lead concentrations near the pXRF IDL. Conversely, onsite results were greater than offsite results in 32 percent (n=72 of 224; table 2.2) of samples. Surficial dust and the presence of more, smaller-sized particles (which are typically associated with greater metals concentrations) at the undisturbed soil surface, or even surface geometry, were at least partially responsible. Further, it is likely that smaller-sized particles migrated to the bottom of the bags where they were less accessible for offsite quantification (that is, due to the limited depth sampling of the pXRF method).
Comparison of Lead Concentrations in Soil by Onsite pXRF and ICP–MS
A total of 47 soil samples had onsite pXRF and ICP–MS results for lead. RPDs between the two results ranged from 2.5 to 127 percent, and the mean RPD±SE was 40.2±4.3 percent (table 2.2). Linear regression of the results for the two different methodologies had R2=0.878 at a 95-percent confidence level (fig. 2.1C); and Spearman’s correlation coefficient was 0.874 (probability value was <0.001).
Use of Combined Onsite and Offsite pXRF Data for Site Characterization
Having generally validated the offsite pXRF results with ICP–MS (R2=0.986) but given the method and sensitivity differences between ICP–MS and pXRF, we decided to use only pXRF data to characterize the local lead concentrations in soil at our study sites. Evaluations of avian reproductive metrics were made using lead concentrations in soil measured by onsite pXRF only, offsite pXRF only, and using combined onsite and offsite data; these evaluations yielded very similar outcomes in terms of correlations of bird reproduction success with lead concentration in soil. Therefore, we pooled onsite and offsite pXRF data; however, it is important to note that offsite pXRF results for duplicate samples were not included in the pool. Rather, duplicates were considered QC samples only. We felt that having a greater density of (pooled onsite and offsite) pXRF results, and thus greater spatial coverage, would provide a better representation of the ranges of lead concentrations in soil to which songbirds might be exposed. Also, we note that because of wet field conditions, nearly all the soil samples for the 2018 field season were collected and analyzed via offsite pXRF. Therefore, excluding offsite pXRF data from the analyses would have reduced the density of the data used to estimate local lead concentration for each bird nest, particularly for 2018 nests, which were more distant from nests monitored in 2016 and 2017. Ultimately, we believe that the onsite and offsite pXRF concentration results, although not always in full agreement, still presented a reasonably accurate portrait of songbird exposure to lead at the study sites based on the gradient of field conditions to which the birds would be exposed to on a daily or even hourly basis.
References Cited
Cleveland, D.M., Rattner, B.A., Karouna-Renier, N.K., and Lankton, J.S., 2023, Breeding songbird tissue analysis and metal concentrations in tissues, soil and invertebrates collected near nesting sites within the Southeast Missouri Lead Mining District, 2016–19: U.S. Geological Survey data release, https://doi.org/10.5066/P9RV1D60.
Ravansari, R., Wilson, S.C., and Tighe, M., 2020, Portable X-ray fluorescence for environmental assessment of soils—Not just a point and shoot method: Environment International, v. 134, p. 105250. [Also available at https://doi.org/10.1016/j.envint.2019.105250.]
Sahraoui, H., and Hachicha, M., 2017, Effect of soil moisture on trace elements concentrations using portable x-ray fluorescence spectrometer: Journal of Fundamental and Applied Sciences, v. 9, no. 1, p. 468–484, accessed June 2022 at https://doi.org/10.4314/jfas.v9i1.26.
Stone, M., and Droppo, J.G., 1996, Distribution of lead, copper and zinc in size-fractionated river bed sediment in two agricultural catchments of southern Ontario, Canada: Environmental Pollution, v. 93, no. 3, p. 353–362, accessed June 2023. 10.1016/S0269-7491(96)00038-3.
U.S. Environmental Protection Agency [EPA], 1996, Acid digestion of sediments, sludges, and soils [method 3050B, rev. 2]: U.S. Environmental Protection Agency, accessed April 2020 at https://www.epa.gov/esam/epa-method-3050b-acid-digestion-sediments-sludges-and-soils.
U.S. Environmental Protection Agency [EPA], 2007, Field portable x-ray fluorescence spectrometry for the determination of elemental concentrations in soil and sediment [method 6200, rev. 0]: U.S. Environmental Protection Agency, 32 p., accessed September 2021 at https://www.epa.gov/sites/default/files/2015-12/documents/6200.pdf.
Appendix 3. Co-Occurring Metals of Concern for Birds in the Southeast Missouri Lead Mining DistrictByGiven (First) Names: DanielleCleveland and Given (First) Names: David E.Mosby
Introduction
In the Southeast Missouri Lead Mining District, and similarly in other mining districts worldwide, the lead ores are intergrown with suites of co-occurring metals that may be as or more toxic than lead to ecological receptors; in this way, concentrations of multiple metals in soil at mining and milling sites are generally elevated. The focus of this study was on the potential toxicological effects of lead on birds. However, other co-occurring ore body metals also may have additive toxicological effects, and synergistic or antagonistic effects on lead bioavailability and toxicology (for example, additive toxicity of lead and zinc; Konar and Mullick, 1993). Other metals of concern at one or more of the three contaminated study sites (Big River floodplain, Anschutz Mine, Magmont Vent) characterized in soil in the study were zinc, cadmium, copper, nickel, and cobalt.
The Big River drains the Old Lead Belt; and soils within the Big River floodplain have been contaminated by mine tailings that have been washed downstream and dispersed via wind and rain events from multiple mining districts (Noerpel and others, 2020). At least six major tailings piles contribute to contamination along the banks of the Big River; these tailings contain, at minimum, residual lead, zinc, and cadmium. The Magmont Vent study site (Bixby, Mo.; Viburnum Trend) was named for a mine air vent shaft next to the inactive Magmont Mine; ores from the Magmont Mine produced concentrates of lead, zinc, and copper (Wharton and others, 1975). Ores from the Anschutz Mine (Fredericktown, Mo.) contained lead, cadmium, zinc, copper, cobalt, and nickel; and, in fact, the site was recently purchased to restart cobalt extraction. Our three reference study sites (Hawn State Park, Johnson’s Shut-ins State Park, and Cape Girardeau) were removed from known sources of metal contamination. This appendix presents the measured concentrations of zinc, cadmium, copper, nickel, and cobalt in soils and invertebrates, and concentrations of zinc, copper, and cadmium in a subset of avian blood samples at our study sites. We also briefly discuss the potential implications of the presence of those metals on avian toxicology and lead bioavailability.
Cadmium, a renal toxicant and known carcinogen, is not an essential nutrient and is known to biomagnify in liver and kidney tissues of exposed animals (Eisler, 1985; Wayland and Scheuhammer, 2011; Binkowski and Sawicka-Kapusta, 2015). Exposure to cadmium may also produce cardiovascular, hematological, neurological and testicular effects (Agency for Toxic Substances and Disease Registry [ATSDR], 2012). Cadmium in blood can activate and inhibit δ-aminolevulinic acid dehydratase (δALAD) activity, depending on concentration; however, a 50-percent δALAD inhibition by cadmium requires a cadmium concentration 20 times greater than lead (Davis and Avram, 1978). Birds primarily accumulate cadmium via chronic dietary uptake (Scheuhammer, 1987; García-Fernández and others, 1995; Burger, 2008). Intestinal absorption of cadmium can be enhanced when dietary uptake of essential nutrients, including calcium, is insufficient (Koo and others, 1978). Cadmium also reduces the intestinal absorption of essential elements such as calcium, iron, and zinc. In contrast, dietary selenium has reduced the concentration of cadmium in liver tissues (Li and others, 2013).
Recorded effects of cadmium exposure on birds include intestinal damage and defects (for example, lesions and hypertrophy), kidney and liver damage (for example, inflammatory responses, fibrosis, necrosis, and mineralization of proximal tubules), skeletal effects (for example, inhibition of bone matrix formation and reduced bone mineral content in chicks), induction of oxidative stress, decreased metabolism, endocrine disruption, and deleterious changes to reproductive organs. Liver and kidney tissues account for 67 to 97 percent of the total body burden of cadmium in birds; similar to lead, concentrations of cadmium in the liver and kidney likely reflect chronic exposure, whereas cadmium in blood likely reflects recent exposure (Friberg and others, 1986). Birds possess several mechanisms of cadmium elimination, including metallothionein induction, gut clearance, feather growth, and uropygial gland secretion. Table 3.1 summarizes the estimated background concentrations of cadmium in bird kidneys, livers, and blood (adapted from Wayland and Scheuhammer, 2011); table 3.1 also provides estimated ranges for toxicity thresholds for birds and lists effects that may occur in birds when cadmium concentrations exceed the threshold ranges in those tissues.
Table 3.1.
Background and threshold concentrations for cadmium, zinc, copper, cobalt, and nickel in adult birds.[≥, greater than or equal to; <, less than; >, greater than; --, no data]
Tissue-based concentrations for birds were converted from a wet-weight basis to a dry-weight basis assuming the following tissue moisture contents: blood: 78.26 percent moisture; liver: 67.74 percent moisture; kidney: 76.74 percent moisture (Franson and Pain, 2011).
Background tissue and threshold concentrations are reproduced from literature surveys compiled by Eisler (1993; 1998a, b; copper, nickel, and zinc), Wayland and Scheuhammer (2011; cadmium), or represent concentrations measured in tissues of reference birds in the Southeast Missouri Lead Mining District (for background blood concentrations of cadmium, zinc, and copper; Beyer and others, 2013). Soil background concentration ranges represent Missouri-specific values (Beyer and others, 2013). Soil threshold concentrations represent southeast Missouri-specific values as proposed in EPA (2005a, b, 2006a, b, 2007, 2020), or benchmarks for protection of American robins (Beyer and Sample, 2017). Missouri-specific soil threshold ranges for the protection of vermivores (American robins or American woodcocks), if available, are presented in parentheses after the literature-based threshold range.
A previous study in the Southeast Missouri Lead Mining District (Beyer and others, 2013) examined concentrations of cadmium in blood, liver, and kidneys of adult birds, including Turdus migratorius (Linnaeus, 1766) (American robin), Cardinalis cardinalis (Linnaeus, 1758) (northern cardinal), Molothrus ater (Boddaert, 1783) (brown-headed cowbird), and Pipilo erythrophthalmus (Linnaeus, 1758) (eastern towhee), at reference and mining-contaminated sites. Concentrations of cadmium in soil (Beyer and others, 2013) were 0.09 to 0.69 milligram per kilogram on a dry-weight basis (mg/kg dw) at the reference sites and 1.7 to 9.7 mg/kg dw at the contaminated sites. No concentrations of cadmium in blood samples at either site type (reference or contaminated; 0.001 to 0.063 mg/kg dw) exceeded the 1.2 mg/kg dw threshold (table 3.1). Further, all reference and contaminated livers and kidneys had cadmium concentrations (0.05 to 119 mg/kg dw) within literature-based background ranges for those tissues (table 3.1).
A bird study from another lead-zinc mining district in Missouri (Tri-State Mining District; Beyer and others, 2004) also examined the uptake of cadmium (as a co-occurring ore body metal) in tissues from Zenaida macroura (Linnaeus, 1758) (mourning doves), Riparia riparia (Linnaeus, 1758) (bank swallows), American robins, and northern cardinals. Although the cadmium concentrations in the tissues were generally greater in birds captured at the contaminated sites (blood, less than [<] 0.1 to 0.18 mg/kg dw; liver, 0.91 to 5.9 mg/kg dw; kidney, 0.65 to 33 mg/kg dw) compared to the reference sites (blood, 0.15 to 0.2 mg/kg dw; liver, 0.33 to 2.8 mg/kg dw; kidney, 0.066 to 90 mg/kg dw); none of the concentrations in the passerines or doves exceeded toxicity thresholds (table 3.1).
Zinc, copper, and cobalt are essential nutrients and can generally be physiologically regulated at low tissue concentrations (for example, via homeostasis mechanisms). Conversely, these metals can be toxic to animals at elevated concentrations. Zinc is important in enzyme production, immune and metabolic function, and hormone activity, but excess concentrations of dietary zinc can cause anemia, gastrointestinal irritation, and pancreatic and adrenal abnormalities (Park and others, 2004; ATSDR, 2005a). For example, zinc-induced pancreatitis has been documented in Branta canadensis (Linnaeus, 1758) (Canada geese) in the Tri-State Mining District (Sileo and others, 2003; van der Merwe and others, 2011). A balance exists between zinc sufficiency and deficiency; for example, avian diets should contain greater than (>) 25 mg/kg dw of zinc to prevent zinc deficiency effects but <178 mg/kg dw to prevent sublethal effects (Eisler, 1993). Interestingly, zinc activates δALAD and prevents δALAD inhibition (for example, Hutton, 1983; Bernard and Lauwerys, 1987), except at substantially elevated concentrations of zinc in the blood (>955 micromole per liter [μmol/L]; Thompson and others, 1977). The kidney, pancreas, and bones are typically targeted for toxicity by zinc. Zinc concentrations in bird tissues are typically <210 mg/kg dw in birds; and zinc poisoning generally occurs in birds with liver or kidney concentrations >2,100 mg/kg dw of zinc (table 3.1; Eisler, 1993). Whole blood levels exceeding 2 mg/kg are generally indicative of toxicosis, and a blood reference range for zinc is 1 to 2 mg/kg in parrots (Osofsky and others, 2001). However, tissue concentrations of zinc are generally inconsistent predictors of zinc toxicoses because of its regulation via homeostasis (Howard, 1992; Eisler, 1993).
Beyer and others (2013) previously measured zinc in soils and bird tissues in the Southeast Missouri Lead Mining District. Concentrations of zinc in reference soils were 39 to 149 mg/kg dw; soils from contaminated sites had zinc concentrations ranging from 53 to 1,020 mg/kg dw. Mean concentrations (plus or minus [±] standard error [SE]) of zinc in all bird species were the following: blood, reference=21.8±0.8 mg/kg dw, contaminated=22.4±1.5 mg/kg dw; liver, reference=99.6 ± 2.8 mg/kg dw, contaminated=95.9±3.4 mg/kg dw; and kidney, reference=92.7±2.3 mg/kg dw, contaminated=94.3±2.4 mg/kg dw. Tissue concentrations of zinc at contaminated sites were not different from concentrations of zinc in reference tissues.
Copper is essential for enzyme production, hemoglobin synthesis, and normal growth and metabolic function, but like zinc, dietary and tissue-based concentrations of copper can be deficient, sufficient, or in-excess. Excess copper has been documented to cause decreased hemoglobin and renal damage in rats (ATSDR, 2004a), and can inhibit growth and reduce egg hatchability in birds (Eisler, 1998a; Gou and others, 2021). Mu and others (2019) and Fei and others (2019) demonstrated that copper sulfate dosed to chickens (at 300 mg/kg for 12 weeks) caused mitochondrial dysfunction and lesions in the gizzard and glandular stomach. Copper exposure has also been shown to inhibit δALAD (Thompson and others, 1977) and induce oxidative stress in chickens (Sun and others, 2018). Eeva and Lehikoinen (1996) found depressed fledging success in passerine species Parus major (Linnaeus, 1758) (great tits) and Ficedula hypoleuca (Pallas, 1764) (pied flycatchers) that was associated with nesting distance from a copper smelter in Finland. Fledging success of the great tits increased with increasing distance from the smelter; the pied flycatcher saw adverse effects (that is, lowered hatchability, increased nestling mortality) at the location closest to the smelter. Those authors (Eeva and Lehikoinen, 1996) also evaluated concentrations of metals (copper, nickel, and lead) in bird feces as evidence of exposure and found that concentrations of the metals were markedly elevated within 1.5 kilometers of the smelter but decreased with increasing distance from the smelter.
Cobalt is an essential trace element that is a component of vitamin B12 (cobalamin); cobalamin is necessary for nucleic acid synthesis and metabolism; deficiency in chickens is associated with slowed growth, poor feathering, and reduced hatchability (for example, Skinner and others, 1951; Mariakulandai and McGinnis, 1953). However, excessive cobalt exposure increases red cell mass and production of erythropoietin, depresses enzyme activity, and is also linked to cardiomyopathy and reproductive effects in rats (Paternain and others, 1988; Pedigo and Vernon, 1993; Haga and others, 1996). Exposure to soluble forms of cobalt increases oxidative stress and induces deoxyribonucleic acid damage (ATSDR, 2004b). Gallus gallus (Linnaeus, 1758) (chickens) fed diets having 250 mg/kg or 500 mg/kg cobalt developed pancreatic fibrosis, hepatic necrosis, and other lesions; other effects included ventricular hypertrophy or failure, and accumulation of fluid in the peritoneal cavity (Diaz and others, 1994). Cobalt can also displace zinc from zinc-required enzymes (Gad, 2014). The European Chemicals Agency (2021) has assigned a dietary no-effects-concentration of 50 mg/kg cobalt in prey species; however, cobalt has a trophic transfer factor <1 and concentrations are generally controlled by homeostasis mechanisms in birds. Concentrations of cobalt in bird tissues have not been previously quantified in the Southeast Missouri Lead Mining District. However, given the historical and planned cobalt mining activities in the district, birds may be affected by cobalt contamination either directly via uptake and exposure or indirectly via synergisms or antagonisms with other contaminants.
Nickel is considered an essential micronutrient for the health of some bird species (Eisler, 1998b). Dietary deficiencies and excesses of nickel have been observed for birds; and nickel toxicity (reduced growth) can be enhanced by the addition of cobalt to chicken diets (Ling and Leach, 1979). Nickel concentrations are generally elevated in feathers, eggs, and internal tissues of exposed birds (Darolova and others, 1989; Outridge and Scheuhammer, 1993). Nickel begins accumulation in the tissues of Anas platyrhynchos (Linnaeus, 1758) (mallards) at dietary concentrations of 12.5 mg/kg ration; diets containing 800 to 1,200 mg/kg of nickel resulted in metabolic upset, altered bone densities, inhibited growth, and reduced survival (Eisler, 1998b). Similarly, dietary inputs having greater than or equal to 500 mg/kg nickel resulted in reduced growth and survival for some strains of chickens and mallards (Ling and Leach, 1979; Cain and Pafford, 1981). However, the correlation of nickel intoxication and elevated concentrations of nickel in tissues is species-dependent; and tissue-based nickel concentrations may not be indicative of nickel exposure (Outridge and Scheuhammer, 1993). For example, Chau and Kulikovsky-Cordeiro (1995) demonstrated seasonal variations in nickel concentrations in the crop of ruffed grouse (62 mg/kg dw in May; 136 mg/kg dw in September) because of changes in dietary preferences. Other health effects of nickel exposure include induction of oxidative stress, and impairment of the respiratory tract (via inhalation exposure), the immune system, and potentially the reproductive system (ATSDR, 2005b).
Methods
The methods used to collect samples and quantify co-occurring ore body metals, as well as the data handling procedures, were previously described in the main text. Analyses of zinc, cadmium, copper, nickel, and cobalt were performed concurrently with the lead analyses (on the same sample portions). Briefly, we used portable X-ray fluorescence spectrometry (pXRF, both onsite and offsite approaches) to quantify metals in soils. The measurement units for pXRF were parts per million (ppm), where 1 ppm is approximately equivalent to 1 mg/kg; metals in invertebrates (zinc, cadmium, nickel, cobalt, and copper) and bird blood (zinc, cadmium, and copper) were quantified using inductively coupled plasma-mass spectrometry (ICP–MS) with microwave-assisted digestion in nitric acid and hydrogen peroxide; concentrations are in milligrams per kilogram on dry-weight basis. Instrument detection limits for the pXRF systems and estimated limits of quantification for the ICP–MS method are given in table 3.2.
Table 3.2.
Estimated limits of quantification and reporting limits.[The ICP–MS data are summarized from Cleveland and others (2023). pXRF, portable X-ray fluorescence spectrometry; IDL, instrument detection limit; ppm, part per million; LOQ, estimated limit of quantification; ICP–MS, inductively coupled plasma-mass spectrometry; mg/kg dw, milligram of analyte per kilogram of sample on a dry-weight basis]
Results
Soils
Zinc was the most frequently detected co-occurring ore body metal (table 3.3); concentrations of zinc in soils ranged from below the pXRF instrument detection limit (<18 ppm; instrument detection limit=18 to 23 ppm) to 6,060 ppm. The Big River floodplain had the greatest mean concentration of zinc in soil (348 ppm), but the greatest maximum concentration of zinc at a single sampling point was measured in soil at Magmont Vent (6,060 ppm). Cadmium concentrations in soils were generally below the instrument detection limit for pXRF (14 ppm); cadmium was not detected at the reference sites (table 3.3). Concentrations of cadmium in soil were also generally low at Anschutz Mine and Big River floodplain (<30 ppm); Magmont Vent soils had a maximum concentration of 85 ppm of cadmium.
Table 3.3.
Concentrations of co-occurring ore body metals in soils collected from contaminated and reference study sites in the Southeast Missouri Lead Mining District.[ND, not detected (below a manufacturer-set instrument detection limit)]
Concentrations were measured using a portable X-ray fluorescence spectrometer (pXRF) using onsite and offsite measurement methods. The Kaplan-Meier method was used to present the extremes of the mean when one or more elemental concentration in the dataset was below the instrument detection limit (IDL; table 3.2). The near-zero value used for the Kaplan-Meier mean calculations were 1 part per million (ppm) for zinc, nickel, and cobalt; 0.1 ppm for cadmium; and 5 ppm for copper; and the IDLs were 13 ppm for zinc and cobalt; 4 ppm for cadmium and nickel; and 18 ppm for copper. Soils analyzed on site by pXRF were not dried and were measured as-is; soils analyzed using offsite pXRF were sieved and dried before analyses. Onsite analyses were not performed if the area of soil was too wet (that is, visible water or puddles were within 1 meter of the location).
Concentrations of nickel and cobalt in soils at the reference sites were generally low, with few positive detections; copper concentrations were not recorded for Hawn State Park or Johnson’s Shut-ins State Park. Mean soil concentrations of copper at Anschutz Mine were an order of magnitude greater for copper, and nearly two orders of magnitude greater for nickel and cobalt, than mean concentrations at the other two contaminated sites (that is, Magmont Vent and Big River floodplain; table 3.3). The maximum concentrations of copper, nickel, and cobalt in soils at Anschutz Mine were 20,700 ppm; 12,300 ppm; and 11,200 ppm, respectively; Kaplan-Meier mean concentrations ranged from 71 to 720 ppm for copper, 524 to 526 ppm for nickel, and 838 to 839 ppm for cobalt. In contrast, the greatest mean concentrations for copper, nickel, and cobalt, respectively, for the other sites were 70.1 ppm (Magmont Vent), 8.1 ppm (Magmont Vent), and 17.6 ppm (Big River floodplain).
Invertebrates
Concentrations of co-occurring ore body metals in nondepurated invertebrates (90 percent earthworms, 10 percent beetle grubs by weight) generally varied by site (table 3.4). Zinc concentrations ranged from 150 to 328 mg/kg dw at the reference sites and from 197 to 1,020 mg/kg dw at the contaminated sites. Concentrations of cadmium in invertebrate tissues were 1.29 to 6.34 mg/kg dw at the reference sites and 2.36 to 237 mg/kg dw at the contaminated sites. Mean cadmium concentrations at Big River floodplain and Magmont Vent were at least 20 times greater than mean cadmium concentrations at Anschutz Mine and the reference sites. Nickel concentrations at the reference sites were 3.85 to 8.03 mg/kg dw; nickel concentrations in invertebrates from contaminated sites were 1.59 to 456 mg/kg dw. The mean nickel concentration in invertebrates from Anschutz Mine was 29 times greater, at minimum, than at all other sites (reference or contaminated). Invertebrate concentrations of cobalt were 3.66 to 8.17 mg/kg dw at the reference sites and 2.93 to 384 mg/kg dw at the contaminated sites. The trend for cobalt was similar to that of nickel; and the mean concentration of cobalt in invertebrates from Anschutz Mine was at least 23 times greater than mean concentrations of cobalt from all other sites (table 3.4). Concentrations of copper in invertebrates were 6.61 to 11.1 mg/kg dw at the reference sites and 9.33 to 2,554 mg/kg dw at the contaminated sites. Mean concentrations of copper in Anschutz Mine invertebrates were 42 to 180 times greater than the mean concentrations of copper at all other sites (reference or contaminated).
Table 3.4.
Concentrations of co-occurring ore body metals in mixed invertebrate samples collected near nesting areas at contaminated and reference sites in the Southeast Missouri Lead Mining District.[Data are summarized from Cleveland and others (2023).]
Samples are composited earthworms and beetle larvae that were not depurated; samples also were not rinsed to remove surficial soils and dusts. Composites consisted of about 90 percent earthworms and 10 percent beetle larvae by wet weight. Concentrations were measured using inductively coupled plasma-mass spectrometry (ICP–MS) following microwave-assisted acid digestion and are reported in milligrams of metal per kilogram of lyophilized invertebrate tissue on a dry-weight (dw) basis. No results were below the limits of quantification (LOQs) for ICP–MS. The LOQs (mg/kg dw) for invertebrates were the following: cobalt, 0.04 to 0.2; nickel, 0.05 to 0.2; copper, 0.2; zinc, 1 to 2; and cadmium, 0.1. Statistical comparisons were not made due to low sample size.
Blood
A subset of a limited number of bird blood samples (adult and brood) was analyzed for concentrations of cadmium, copper, and zinc (table 3.5). Samples from the Cape Girardeau reference site were not analyzed for co-occurring ore body metals; cobalt and nickel were not analyzed in blood. Cadmium concentrations were at or near the method quantification limit for all sites and species. Blood concentrations of zinc and copper were generally on the same order of magnitude across sites within each species. Most notably, the mean concentrations of zinc in broods of Sialia sialis (Linnaeus, 1758) (eastern bluebirds), eastern towhees, Passerina cyanea (Linnaeus, 1766) (indigo buntings), and northern cardinals were 1.3 to 2.1 times greater in broods than those of their adults at the contaminated sites; however, sample sizes were low, which may affect these types of comparisons.
Table 3.5.
Concentrations of co-occurring ore body metals in blood samples from adult birds and their broods at contaminated and reference sites in the Southeast Missouri Lead Mining District.[Data are summarized from Cleveland and others (2023). Concentrations of co-occurring ore body metals were measured in nonlethal blood samples on a dry-weight basis (milligrams per kilogram, mg/kg dw) using inductively coupled plasma-mass spectrometry (ICP–MS); values are mean plus or minus (±) standard error. A value of 0 was substituted for values censored as below the estimated limit of quantification (LOQ) for ICP–MS in the mean and standard error calculations. The LOQs (mg/kg dw) for blood were the following: copper, 0.1 to 0.3; zinc, 2 to 4; and cadmium, 0.02 to 0.06. All collection blanks had concentrations of cadmium, zinc, and copper below the LOQ. Statistical comparisons were not made due to low sample size. --, no data; <, less than]
Discussion
Soils and Invertebrates
As described in the main text, the U.S. Environmental Protection Agency (EPA) performed a site-specific baseline ecological risk assessment (BERA) for the Big River Mine Tailings Site (EPA, 2006a) and an ecological risk assessment (ERA) for the Southwest Jefferson County Mining Site (EPA, 2020) to evaluate potential sources of contamination to the Big River watershed and floodplain. The BERA and Southwest Jefferson County Mining Site ERA used worm tissue, soil, and other media concentrations specific to southeast Missouri to evaluate the potential for adverse effects to birds. It is important to note that the BERA used the Scolopax minor (J. F. Gmelin, 1789) (American woodcock) as the model vermivore species, whereas the Southwest Jefferson County Mining Site ERA used the American robin and the American woodcock. However, toxicity threshold concentrations for robins and woodcocks in the Southwest Jefferson County Mining Site ERA were the same for cadmium and zinc for both species.
The Southwest Jefferson County Mining Site ERA calculated that a concentration of cadmium in soil of 4.94 mg/kg dw (3.1 mg/kg cadmium in floodplain sediments) and a cadmium concentration of 59.1 mg/kg in earthworms would exceed adverse effects thresholds (survival, growth, or reproduction) for birds (EPA, 2020). The BERA toxicity threshold for worms was 113 mg/kg dw of cadmium, and the BERA threshold for soil was 24 mg/kg dw of cadmium (EPA, 2006a). Few concentrations of cadmium in soil exceeded the 24 mg/kg dw threshold (n=7 of 1,105; 0.6 percent) at our study sites, and all exceedances except 1 were in soils from Magmont Vent. The pXRF method that we used for quantification of lead and co-occurring ore body metals in soils cannot reliably quantify concentrations of cadmium in soil below 14 ppm. This pXRF instrument detection limit (14 ppm) is well above the 3.1 mg/kg dw and 4.94 mg/kg dw thresholds established by the Southwest Jefferson County Mining Site ERA for cadmium in sediments and soil. Only 102 of 1,105 soils (9.2 percent) had positive detections for cadmium and exceeded the greater 4.94 mg/kg dw threshold concentration for cadmium by pXRF (table 3.3). However, we quantified cadmium in a subset of soil samples using ICP–MS to validate the pXRF method; ICP–MS is generally more sensitive than pXRF. We found that 40 percent (34 of 84) of soil sample concentrations with cadmium measured by ICP–MS that were collected at the contaminated sites had cadmium concentrations (3.18 to 36.4 mg/kg dw) that exceeded the 3.1 mg/kg dw threshold; there were no exceedances at the reference sites. A total of 3 invertebrate samples had cadmium concentrations that exceeded the 113 mg/kg dw toxicity threshold; these were 2 of 11 at Big River floodplain (18 percent) and 1 of 3 at Magmont Vent (33 percent). The lower Southwest Jefferson County Mining Site ERA threshold (59.1 mg/kg in earthworms) was exceeded by 7 of 9 samples from Big River floodplain (78 percent) and 3 of 3 samples from Magmont Vent (100 percent). No other sites had invertebrate samples with cadmium concentrations exceeding the 59.1 mg/kg dw threshold for earthworms; however, sample size was small.
The Big River BERA (EPA, 2006a) established toxicity thresholds (protective of woodcocks) for zinc in soil (234 mg/kg dw) and earthworms (182 mg/kg dw) for southeast Missouri. Similarly, the thresholds established by the Southwest Jefferson County Mining Site ERA (American robin and American woodcock) were 439 mg/kg dw in soil and 316 mg/kg dw in earthworms. The mean concentrations of zinc in soils at Magmont Vent (344 ppm) and Big River floodplain (348 ppm) sites exceeded the BERA threshold (234 mg/kg dw); mean concentrations of zinc in soils at Anschutz Mine (109 ppm) and the reference sites were below the threshold. However, the maximum concentration of zinc in soils at Anschutz Mine (1,738 ppm; table 3.3) exceeded the BERA threshold concentration by an approximate factor of seven. Maximum concentrations of zinc in soils at Big River floodplain (1,890 ppm) and Magmont Vent (6,060 ppm) exceeded the threshold by factors of about 8 and 26, respectively. Maximum soil concentrations of zinc at the reference sites (82 to 166 ppm; table 3.3) did not exceed the potential adverse effects concentration (234 mg/kg). The mean concentrations of zinc in invertebrates at all sites, reference and contaminated, exceeded the 182 mg/kg dw threshold for woodcock for zinc in earthworms (table 3.4). Only the mean concentrations of zinc at the contaminated sites exceeded the 316 mg/kg dw zinc concentration threshold for American robins, although 1 of 3 individual invertebrate samples at Johnson’s Shut-ins State Park had a zinc concentration >316 mg/kg dw (328 mg/kg dw, table 3.4).
Unfortunately, neither the Big River BERA or the Southwest Jefferson County Mining Site ERA evaluated site-specific toxicity thresholds for copper, nickel, or cobalt in terrestrial receptors in the Southeast Missouri Lead Mining District. Therefore, we used the EPA’s Ecological Soil Screening Levels (Eco-SSLs) to evaluate the potential for adverse effects to birds from exposure to soil-borne copper, nickel, and cobalt. The Eco-SSL values generally provide conservative soil screening benchmarks because the thresholds were derived to avoid underestimating risk, whereas site-specific thresholds account for local soil chemistry, local fauna, and other factors. For example, the Eco-SSL for cadmium in soil is 0.77 mg/kg dw for protection of woodcock (EPA, 2005a); in contrast, the site-specific risk analyses derived cadmium concentrations in soils of 4.94 to 24 mg/kg dw for protection of American robins (EPA, 2020).
Eco-SSL thresholds are typically estimated separately for the insectivorous (woodcock) and herbivorous (dove) avian trophic levels. The Eco-SSL concentrations for copper in soil are 28 mg/kg dw for insectivores and 76 mg/kg dw for herbivores (EPA, 2006b); these concentrations represent the onset of adverse reproductive effects for avian vermivores and herbivores, respectively. The mean concentration of copper in Anschutz Mine soil (713 to 720 ppm; table 3.3) was an order of magnitude greater than the Eco-SSL for herbivores (76 mg/kg dw); and the maximum copper concentration (20,700 ppm) was up to 740 times greater than the Eco-SSL thresholds. The upper range of the mean concentration of copper in Big River floodplain soils (27.3–36.1 ppm) exceeded the copper Eco-SSL for vermivores but did not exceed the herbivore threshold. Soils at Magmont Vent had a mean copper concentration (66.0–70.1 ppm; table 3.3) that exceeded the Eco-SSL thresholds for avian vermivores.
The Eco-SSL concentration for nickel in soil, 210 mg/kg dw, represents the soil concentration at which adverse growth and reproductive effects due to nickel exposure may be experienced by herbivorous avian receptors; however, a threshold could not be determined for insectivores because the EPA could not determine a reliable method to estimate the uptake of nickel by soil invertebrates from soil (EPA, 2007). The mean concentration of nickel in soil at Anschutz Mine (524 to 526 ppm) was 2.5 times greater than the 210 mg/kg dw threshold (table 3.3) and the maximum concentration of nickel in a point sample at Anschutz Mine (12,300 ppm) exceeded the threshold by an approximate factor of 60. No other sites (reference or contaminated) had soil samples with nickel concentrations exceeding the Eco-SSL threshold.
Soil thresholds (Eco-SSLs) for adverse growth effects due to cobalt exposure are 120 mg/kg dw for insectivores and 270 mg/kg dw cobalt for herbivores (EPA, 2005b). The mean concentration of cobalt in soils at Anschutz Mine exceeded both Eco-SSL thresholds values by three to seven times (table 3.3). No other site had a mean concentration of cobalt in soil that exceeded the Eco-SSLs. However, the maximum concentrations of cobalt in soils at Magmont Vent (181 ppm; contaminated) and Big River floodplain (197 ppm; contaminated) exceeded the Eco-SSL threshold for avian vermivores (120 mg/kg dw).
We also noted that the mean concentrations of co-occurring ore body metals in invertebrate tissues were, in some cases, greater than the mean concentration of those metals in soils within the same sites (table 3.4). For example, the mean concentration of cadmium in invertebrates at Magmont Vent was 122 mg/kg dw, whereas the mean concentration of cadmium in soil at the site ranged from 2.7 to 5.2 ppm, and the maximum concentration of cadmium was 85 ppm (table 3.3). These types of intra-site differences in metal concentrations between soil and invertebrate samples may indicate biomagnification of the metals from the soil into the invertebrates. However, some caution should be used during interpretation due to the heterogeneous nature of the contaminated sites. Further, the invertebrates were not depurated or washed to remove surficial soils prior to analysis. Concentrations of zinc in invertebrate tissues were relatively similar among all sites (reference and contaminated; table 3.4); zinc concentrations were likely regulated by homeostatic processes.
Blood
There are few literature-based toxicity thresholds for concentrations of cadmium, zinc, and copper in bird tissues, particularly for blood (table 3.1). We were able to find a blood-based toxicity threshold for cadmium (greater than or equal to 1.2 mg/kg dw, assuming 78.26 percent moisture; Wayland and Scheuhammer, 2011). Also, a previous study in the Southeast Missouri Lead Mining District (Beyer and others, 2013) identified a reference concentration of 0.0063 mg/kg dw cadmium in bird blood. Concentrations of cadmium in the blood of adults and broods in our study (table 3.5) were generally at or below the quantification limit; all concentrations were <0.06 mg/kg dw and below the 1.2 mg/kg dw threshold. Blood-based thresholds for zinc and copper were not available in the literature, but we are able compare our results for zinc and copper from adult reference birds to concentrations previously measured by Beyer and others (2013) in the Southeast Missouri Lead Mining District (table 3.5). The mean reference concentrations of zinc (21.8 mg/kg dw; Beyer and others, 2013) and copper (1.54 mg/kg dw; Beyer and others, 2013) in blood were similar to the concentrations of zinc and copper in all adult blood samples in this study, regardless of site type.
These results were not surprising given the homeostatic mechanisms available to regulate body burdens of cadmium, zinc, and copper. For example, once nutritional requirements for copper and zinc are met, metalloproteins would be expected to bind excess concentrations, which would then be excreted when the gastrointestinal mucosal cells are sloughed (Eisler, 1998a). The comparable and relatively low blood concentrations of zinc, copper, and cadmium in the blood of birds at both reference and contaminated sites likely indicate the physiological regulation of these metals. However, limited sample size may have also affected the comparisons. The observation that brood concentrations of metals tended to be greater than those for adults was also not surprising. The effect of age on concentrations of metals in blood has been previously demonstrated (for example, Lock and others, 1992; Burger and others, 2018); however, sample size may also have affected age comparisons.
Effects of Co-Occurring Ore Body Metals on Lead Bioavailability and Bird Exposure
The interactions between metals are complex and are dependent upon species, organ system, and the concentrations and solubilities (for example, mineralogy) of the interacting metals. Co-occurring metals that are synergistic in their toxic effects at one concentration can be antagonistic at other concentrations and vice versa. For example, zinc and cadmium compete for binding to intracellular sites and proteins, but cadmium has greater binding affinity. In this way, the intestinal absorption of dietary zinc is reduced by cadmium, and greater concentrations of cadmium can be absorbed, circulated, and retained in tissues throughout the body. Conversely, the resulting presence of increased concentrations of free zinc, due to release from the binding sites or by additional zinc supplementation in the diet, can induce increased production of metallothioneins, which immobilize cadmium in the intestine for elimination rather than deposition in the body (Brzóska and Moniuszko-Jakoniuk, 2001). The patterns of accumulation and toxicity from multiple-metal interactions often differ from those produced by a single metal toxicant; and acknowledgement of these inter-metal interactions is crucial to understanding the overall effects of mining on birds in the Southeast Missouri Lead Mining District. The ATSDR conducted extensive literature reviews of metal interactions among (1) cadmium and lead (ATSDR, 2004c) and (2) lead, zinc, and copper (ATSDR, 2004d). However, it is important to note that there is limited information for birds; and much of the information compiled by ATSDR is based on studies in mammals. Lead toxicity was evaluated for multiple endpoints in the presence of elevated concentrations of cadmium (ATSDR, 2004c); five studies concluded that combined exposure to lead and cadmium caused “greater than additive” or synergistic lead toxicity (Der and others, 1976; Fahim and Khare, 1980; Marlowe and others, 1985; Lockett and Leary, 1986; Saxena and others, 1989), whereas nine studies concluded that lead and cadmium toxicities were simply additive (Thawley and others, 1977; Fowler and Mahaffey, 1978; Perry and Erlanger, 1978; Roels and others, 1978; Kopp and others, 1980a, b; Buchet and others, 1981; Mahaffey and others, 1981; Voors and others, 1982). Cobbina and others (2015) also found synergistic effects among lead, cadmium, or copper in rat tissues during low-dose chronic exposures. In contrast, the results of five additional studies (Mahaffey and Fowler, 1977; Perry and Erlanger, 1978; Exon and others, 1979; Mahaffey and others, 1981; Nation and others, 1990) indicated that the toxicities of lead and cadmium were “less than additive” or caused antagonistic effects from combined exposure to cadmium and lead. Some degree of conflict among these conclusions was related to the choice of end point that was evaluated. Therefore, ATSDR (2004c) also evaluated the effect of cadmium on concentrations of lead in tissues; and those results tended to more consistently indicate that cadmium has an antagonistic effect on lead uptake in tissues (Mahaffey and others, 1981; Elsenhans and others, 1987; Nation and others, 1990; Skoczyńska and others, 1994). In some cases, the effect of cadmium on lead uptake was dose dependent, exhibiting synergism at high cadmium doses and antagonism at lower cadmium doses (Exon and others, 1979). In contrast, studies on rat brain tissues have indicated that cadmium had no effect on lead concentrations (Mahaffey and others, 1981; Skoczyńska and others, 1994).
ATSDR (2004d) concluded that zinc and copper had overall less-than-additive effects on lead toxicity when evaluating neurological and hematological endpoints. For example, Flora and others (1991) demonstrated that rats fed diets high in zinc had reduced concentrations of lead in blood, liver, and kidney tissues compared to rats fed diets with low zinc concentrations (due to enhanced urinary excretion of lead); however, lead concentrations in the brain were not affected by dietary zinc concentrations. Dietary zinc has been shown to reduce the inhibitory effect of lead on δALAD activity (Finelli and others, 1975). Batra and others (1998) showed that co-administration of zinc reduced the accumulation of lead in rat testes, thereby reducing the toxicological effects. The effects of copper on the biological uptake and toxicity of lead are also varied. For example, δALAD activity was found to be increased by the presence of elevated concentrations of copper and lead in the diet of rats compared to rats exposed to dietary lead alone (Flora and others, 1982). However, elevated copper concentrations did not affect lead concentrations in blood, bone, liver, and kidney in rats (Cerklewski and Forbes, 1977). In another rat study (Malhotra and others, 1982), a combined diet of lead and elevated copper decreased dopamine concentrations in the brain compared to diets containing either copper or lead. Although the lead-copper mixed metal diet did not affect the concentration of lead in the brain, concentrations of copper in the brain were decreased.
Moreover, synergistic and antagonistic effects are not limited to interactions between lead and a co-occurring ore body metal. Rather, co-occurring metals can affect the behavior and toxicity of other co-occurring metals, which may subsequently affect lead toxicity. Interactions also depend on physicochemical conditions. For example, zinc can behave antagonistically with copper (for example, Ahsanullah and others, 1988; Vranken and others, 1988; Kraak and others, 1994), whereas zinc and cadmium, and zinc and nickel, have additive toxicities (Negilski and others, 1981; Vranken and others, 1988; Kraak and others, 1994). Copper interacts with multiple metals, including zinc, iron, molybdenum, manganese, magnesium, nickel, cadmium, and selenium; these interactions can be beneficial or toxic (Kirchgessner and others, 1979; Flora and others, 1991).
Overall, there appears to be limited data in the scientific literature evaluating the effects of metal mixtures on birds. French and others (2017) provided a brief review of the effects of dietary zinc supplementation on the uptake of lead in birds and concluded that zinc had minimal effect on lead toxicity and absorption. Di Giulio and Scanlon (1984) found that exposure to lead did not significantly affect copper and zinc tissue concentrations in mallards; and Dauwe and others (2002) found that lead affected concentrations of zinc in Taeniopygia guttata (Vieillot, 1817) (zebra finch) feathers, but not zinc concentrations in liver tissues. Blanco and others (2003, 2004) evaluated eggs, blood, and liver tissues to determine the exposure of Milvus migrans (Boddaert, 1783) (black kites) to metals from a solid waste incinerator in Spain; an antagonistic relationship between copper, zinc, and lead concentrations and an antagonistic effect of cadmium with zinc and copper were observed. Wren and others (1994) found positive correlations between copper and cadmium, and copper and zinc, in Lagopus lagopus (Linnaeus, 1758) (willow ptarmigan) kidneys; copper and zinc also had positive correlations in the kidneys and livers of Uria aalge (Pontoppidan, 1763) (common murres; Stewart and others, 1994). The sample size for our blood dataset (table 3.5) is likely too small to draw any definitive conclusions about metal interactions and subsequent health effects.
Bird exposure and uptake of lead may also be affected by local lead bioavailability and differences in soil chemistry (for example, Ming and others, 2012). The bioavailabilities, solubilities, and speciation of the co-occurring ore body metals also affect their uptake and ability to interact with lead and one another. The soils at our study sites (reference and contaminated) in southeastern Missouri were weathered from carbonate rock, and bioavailability studies with that Missouri soil type have consistently demonstrated relatively high lead bioavailability (Yang and others, 2002; Casteel and others, 2006; Weber and others, 2015; Beyer and others, 2016). However, lead speciation, solubility, and ultimately bioavailability, may also differ as a function of the source of the lead contamination (for example, mine/mill waste versus smelter waste) and can be further complicated by fluvial transport, oxidation/reduction cycles, and differential weathering. Other bioavailability factors include soil pH, the speciation and concentration of natural organic matter and other ligands in soils, and the particle size distributions of the soils (for example, Quenea and others, 2009).
Although lead speciation is beyond the scope of this study, differences in concentrations of other dominant divalent metals in site soils may have affected lead uptake from site soils into birds. We calculated ratios between concentrations of lead and the additive concentrations of the two (zinc and copper) or three (zinc, copper, and nickel) predominant co-occurring ore body metals for each site. Metal prevalence was based on (1) the frequency of positive detection by pXRF and (2) metals having the greatest concentrations at the contaminated sites. The calculated ratios indicate the relative prevalence of lead within the soils at each contaminated site relative to the other divalent metals. In cases where the ratio of lead to co-occurring metals is <1, there may be more opportunity for adjustments to lead toxicity due to the presence of other metals, whereas ratios >1 may indicate that lead toxicity is less likely to be affected by synergisms or antagonisms from co-occurring metals. The ratios have been calculated according to equation 1.1 (three metals) and equation 1.2 (two metals); the square brackets (that is, []) in the equations below indicate the mean soil concentration of the metal indicated (table 3.3); the colon (that is, :) represents the ratio of lead to the other metals. The concentrations of lead in soil were previously given in the main text (table 6).
whereΣ
is the sum of concentrations,
Zn
is the mean zinc concentration,
Cu
is the mean copper concentration, and
Ni
is the mean nickel concentration.
The calculated ratios (table 3.6) indicate that potential additive, antagonistic, or synergistic effects on lead toxicity might be expected to be most prominent at Anschutz Mine, due to elevated soil concentrations of zinc, copper, and nickel relative to those at Big River floodplain and Magmont Vent (table 3.3). Conversely, soil chemistry at Magmont Vent might be expected to have the lowest effect (either antagonistic or synergistic) on lead uptake compared to the other sites, due to the elevated concentration of lead in soil (relative to the other metals) at that site compared to the other two contaminated sites. However, these comparisons are limited by the relatively high instrument detection limit (14 ppm) of pXRF for cadmium, which had the effect of decreasing the number of samples with quantifiable results. Addition of cobalt concentrations to the equations might demonstrate further effects on lead toxicity, particularly at Anschutz Mine.
Table 3.6.
Ratios of lead to co-occurring divalent metals in soils.[Ratios of lead to co-occurring ore body metals were calculated according to equations 1.1 and 1.2 and indicate the relative prevalence of lead within the soils at each contaminated site relative to the other metals in site soils. Σ, sum of mean concentrations; Cu, copper; Ni, nickel; Zn, zinc]
Conclusions
These results, together with the findings for lead in the main text, indicate that birds breeding in the Southeast Missouri Lead Mining District have been exposed to and taken up lead, zinc, cadmium, nickel, cobalt, and copper from soil and invertebrates; and the specific metal mixtures and metal concentrations vary by site and species. Concentrations of cadmium, zinc, copper, nickel, and cobalt in soil or in invertebrates exceeded protective thresholds at multiple sites. Exceedances in soil occurred at Magmont Vent (cadmium, zinc, copper, and cobalt), Big River floodplain (zinc, copper, and cobalt), and Anschutz Mine (zinc, copper, nickel, and cobalt); there were no exceedances at the reference sites. Concentrations of cadmium and zinc in invertebrates also exceeded avian toxicity thresholds (cadmium, Big River floodplain, Magmont Vent; zinc, all sites). The results for invertebrates also suggested the potential for biomagnification of cadmium. No concentrations of cadmium in blood samples exceeded the 1.2 mg/kg dw threshold; however, it may be instructive to expand future studies of co-occurring ore body metals to include liver and kidney tissues to provide a snapshot of chronic avian exposure to metals other than lead.
It is also likely that birds in the Southeast Missouri Lead Mining District have been exposed to co-occurring metals in soils at the contaminated sites at concentrations that (1) themselves induce toxicity, or (2) enhance the toxicological effects of lead, particularly at Anschutz Mine. Based solely on relative concentrations of the metals in soils, the potential for additional adverse effects would most likely be due to cadmium and zinc at the Big River floodplain and Magmont Vent sites, and due to copper, cobalt, and nickel at Anschutz Mine. Moreover, it is likely that interactions among the metals found in soils at these sites also affect lead bioavailability. However, it is difficult to definitively determine whether these interactions are additive, synergistic, or antagonistic to lead bioavailability and toxicity in avian receptors; further studies of metal speciation, solubility, and interactions specifically in birds may be useful for land managers.
References Cited
Agency for Toxic Substances and Disease Registry [ATSDR], 2004a, Toxicological profile for copper: Atlanta, Ga., U.S. Department of Health and Human Services Public Health Service, accessed August 2020 at https://www.atsdr.cdc.gov/toxprofiles/tp132.pdf.
Agency for Toxic Substances and Disease Registry [ATSDR], 2004b, Toxicological profile for cobalt: Atlanta, Ga., U.S. Department of Health and Human Services Public Health Service, accessed August 2020 at https://www.atsdr.cdc.gov/toxprofiles/tp33.pdf.
Agency for Toxic Substances and Disease Registry [ATSDR], 2004c, Interaction profile for: arsenic, cadmium, chromium, and lead: Atlanta, Ga., U.S. Department of Health and Human Services Public Health Service, accessed August 2020 at https://www.atsdr.cdc.gov/interactionprofiles/ip-metals1/ip04.pdf.
Agency for Toxic Substances and Disease Registry [ATSDR], 2004d, Interaction profile for: lead, manganese, zinc, and copper: Atlanta, Ga., U.S. Department of Health and Human Services Public Health Service, accessed August 2020 at https://www.atsdr.cdc.gov/interactionprofiles/ip-metals2/ip06.pdf.
Agency for Toxic Substances and Disease Registry [ATSDR], 2005a, Toxicological profile for zinc: Atlanta, Ga., U.S. Department of Health and Human Services Public Health Service, accessed August 2020 at https://www.atsdr.cdc.gov/toxprofiles/tp60.pdf.
Agency for Toxic Substances and Disease Registry [ATSDR], 2005b, Toxicological profile for nickel: Atlanta, Ga., U.S. Department of Health and Human Services Public Health Service, accessed August 2020 at https://www.atsdr.cdc.gov/toxprofiles/tp15.pdf.
Agency for Toxic Substances and Disease Registry [ATSDR], 2012, Toxicological profile for cadmium: Atlanta, Ga., U.S. Department of Health and Human Services Public Health Service, accessed August 2020 at https://www.atsdr.cdc.gov/toxprofiles/tp5.pdf.
Ahsanullah, M., Mobley, M.C., and Rankin, P., 1988, Individual and combined effects of zinc, cadmium and copper on the marine amphipod Allorchestes compressa: Marine and Freshwater Research, v. 39, no. 1, p. 33–37. [Also available at https://doi.org/10.1071/MF9880033.]
Batra, N., Nehru, B., and Bansal, M.P., 1998, The effect of zinc supplementation on the effects of lead on the rat testis: Reproductive Toxicology (Elmsford, N.Y.), v. 12, no. 5, p. 535–540. [Also available at https://doi.org/10.1016/S0890-6238(98)00030-6.]
Bernard, A., and Lauwerys, R., 1987, Metal-induced alterations of δ-aminolevulinic acid dehydratase: Annals of the New York Academy of Sciences, v. 514, no. 1, p. 41–47. [Also available at https://doi.org/10.1111/j.1749-6632.1987.tb48759.x.]
Beyer, W.N., Dalgarn, J., Dudding, S., French, J.B., Mateo, R., Miesner, J., Sileo, L., and Spann, J., 2004, Zinc and lead poisoning in wild birds in the Tri-State Mining District (Oklahoma, Kansas, and Missouri): Archives of Environmental Contamination and Toxicology, v. 48, no. 1, p. 108–117. [Also available at https://doi.org/10.1007/s00244-004-0010-7.]
Beyer, W.N., Franson, J.C., French, J.B., May, T., Rattner, B.A., Shearn-Bochsler, V.I., Warner, S.E., Weber, J., and Mosby, D., 2013, Toxic exposure of songbirds to lead in the Southeast Missouri Lead Mining District: Archives of Environmental Contamination and Toxicology, v. 65, no. 3, p. 598–610. [Also available at https://doi.org/10.1007/s00244-013-9923-3.]
Beyer, W.N., Basta, N.T., Chaney, R.L., Henry, P.F.P., Mosby, D.E., Rattner, B.A., Scheckel, K.G., Sprague, D.T., and Weber, J.S., 2016, Bioaccessibility tests accurately estimate bioavailability of lead to quail: Environmental Toxicology and Chemistry, v. 35, no. 9, p. 2311–2319. [Also available at https://doi.org/10.1002/etc.3399.]
Beyer, W.N., and Sample, B.E., 2017, An evaluation of inorganic toxicity reference values for use in assessing hazards to American robins (Turdus migratorius): Integrated Environmental Assessment and Management, v. 13, no. 2, p. 352–359. [Also available at https://doi.org/10.1002/ieam.1792.]
Binkowski, L.J., and Sawicka-Kapusta, K., 2015, Cadmium concentrations and their implications in mallard and coot from fish pond areas: Chemosphere, v. 119, p. 620–625. [Also available at https://doi.org/10.1016/j.chemosphere.2014.07.059.]
Blanco, G., Frías, O., Jiménez, B., and Gómez, G., 2003, Factors influencing variability and potential uptake routes of heavy metals in black kites exposed to emissions from a solid waste incinerator: Environmental Toxicology and Chemistry, v. 22, no. 11, p. 2711–2718. [Also available at https://doi.org/10.1897/02-519.]
Blanco, G., Jiménez, B., Frías, O., Millan, J., and Dávila, J.A., 2004, Contamination with nonessential metals from a solid-waste incinerator correlates with nutritional and immunological stress in prefledgling black kites (Milvus migrans): Environmental Research, v. 94, no. 1, p. 94–101. [Also available at https://doi.org/10.1016/S0013-9351(03)00120-8.]
Brzóska, M.M., and Moniuszko-Jakoniuk, J., 2001, Interactions between cadmium and zinc in the organism: Food and Chemical Toxicology, v. 39, no. 10, p. 967–980. [Also available at https://doi.org/10.1016/S0278-6915(01)00048-5.]
Burger, J., 2008, Assessment and management of risk to wildlife from cadmium: Science of the Total Environment, v. 389, no. 1, p. 37–45. [Also available at https://doi.org/10.1016/j.scitotenv.2007.08.037.]
Burger, J., Mizrahi, D., Tsipoura, N., Jeitner, C., and Gochfeld, M., 2018, Mercury, lead, cadmium, cobalt, arsenic and selenium in the blood of semipalmated sandpipers (Calidris pusilla) from Suriname, South America—Age-related differences in wintering site and comparisons with a stopover site in New Jersey, USA: Toxics, v. 6, no. 2, article number 27. [Also available at https://doi.org/10.3390/toxics6020027.]
Cain, B.W., and Pafford, E.A., 1981, Effects of dietary nickel on survival and growth of mallard ducklings: Archives of Environmental Contamination and Toxicology, v. 10, no. 6, p. 737–745. [Also available at https://doi.org/10.1007/BF01054857.]
Casteel, S.W., Weis, C.P., Henningsen, G.M., and Brattin, W.J., 2006, Estimation of relative bioavailability of lead in soil and soil-like materials using young swine: Environmental Health Perspectives, v. 114, no. 8, p. 1162–1171. [Also available at https://doi.org/10.1289/ehp.8852.]
Cerklewski, F.L., and Forbes, R.M., 1977, Influence of dietary copper on lead toxicity in the young male rat: The Journal of Nutrition, v. 107, no. 1, p. 143–146. [Also available at https://doi.org/10.1093/jn/107.1.143.]
Chau, Y.K., and Kulikovsky-Cordeiro, O.T.R., 1995, Occurrence of nickel in the Canadian environment: Environmental Reviews, v. 3, no. 1, p. 95–120. [Also available at https://doi.org/10.1139/a95-004.]
Cleveland, D.M., Rattner, B.A., Karouna-Renier, N.K., and Lankton, J.S., 2023, Breeding songbird tissue analysis and metal concentrations in tissues, soil and invertebrates collected near nesting sites within the Southeast Missouri Lead Mining District, 2016–19: U.S. Geological Survey data release, https://doi.org/10.5066/P9RV1D60.
Cobbina, S.J., Chen, Y., Zhou, Z., Wu, X., Feng, W., Wang, W., Mao, G., Xu, H., Zhang, Z., Wu, X., and Yang, L., 2015, Low concentration toxic metal mixture interactions—Effects on essential and non-essential metals in brain, liver, and kidneys of mice on sub-chronic exposure: Chemosphere, v. 132, p. 79–86. [Also available at https://doi.org/10.1016/j.chemosphere.2015.03.013.]
Dauwe, T., Bervoets, L., Blust, R., and Eens, M., 2002, Tissue levels of lead in experimentally exposed zebra finches (Taeniopygia guttata) with particular attention on the use of feathers as biomonitors: Archives of Environmental Contamination and Toxicology, v. 42, no. 1, p. 88–92. [Also available at https://doi.org/10.1007/s002440010295.]
Davis, J.R., and Avram, M.J., 1978, A comparison of the stimulatory effects of cadmium and zinc on normal and lead-inhibited human erythrocytic δ-aminolevulinic acid dehydratase activity in vitro: Toxicology and Applied Pharmacology, v. 44, no. 1, p. 181–190. [Also available at https://doi.org/10.1016/0041-008X(78)90297-1.]
Di Giulio, R.T., and Scanlon, P.F., 1984, Effects of cadmium and lead ingestion on tissue concentrations of cadmium, lead, copper and zinc in mallard ducks: Science of the Total Environment, v. 39, no. 1-2, p. 103–110. [Also available at https://doi.org/10.1016/0048-9697(84)90028-7.]
Diaz, G.J., Julian, R.J., and Squires, E.J., 1994, Cobalt‐induced polycythaemia causing right ventricular hypertrophy and ascites in meat‐type chickens: Avian Pathology, v. 23, no. 1, p. 91–104. [Also available at https://doi.org/10.1080/03079459408418977.]
European Chemicals Agency, 2021, Cobalt—Toxicity to birds: European Chemicals Agency registration dossier, EC no. 231–15–0, CAS no. 7440–48–4, accessed March 16, 2021, at https://echa.europa.eu/registration-dossier/-/registered-dossier/15506/6/4/6.
Eeva, T., and Lehikoinen, E., 1996, Growth and mortality of nestling great tits (Parus major) and pied flycatchers (Ficedula hypoleuca) in a heavy metal pollution gradient: Oecologia, v. 108, no. 4, p. 631–639, accessed August 2020 at https://doi.org/10.1007/BF00329036.
Eisler, R., 1985, Cadmium hazards to fish, wildlife, and invertebrates—A synoptic review: U.S. Fish and Wildlife Service Biological Report 85(1.2), Contaminant Hazard Reviews Report no. 2, 30 p., accessed August 2020 at https://www.pwrc.usgs.gov/eisler/CHR_2_Cadmium.pdf.
Eisler, R., 1993, Zinc hazards to fish, wildlife, and invertebrates: a synoptic review. U.S. Fish and Wildlife Service Biological Report 10, 106 p., accessed August 2020 at https://www.pwrc.usgs.gov/eisler/CHR_26_Zinc.pdf.
Eisler, R., 1998a, Copper hazards to fish, wildlife, and invertebrates—A synoptic review: U.S. Geological Survey, Biological Resources Division, Biological Science Report USGS/BRD/BSR—1998–0002, accessed August 2020 at https://pubs.er.usgs.gov/publication/5200199.
Eisler, R., 1998b, Nickel hazards to fish, wildlife, and invertebrates—A synoptic review: U.S. Geological Survey, Biological Resources Division, Biological Science Report USGS/BRD/BSR—1998–0001, accessed August 2020 at https://pubs.er.usgs.gov/publication/5200209.
Elsenhans, B., Schmolke, G., Kolb, K., Stokes, J., and Forth, W., 1987, Metal-metal interactions among dietary toxic and essential trace metals in the rat: Ecotoxicology and Environmental Safety, v. 14, no. 3, p. 275–287. [Also available at https://doi.org/10.1016/0147-6513(87)90071-6.]
Exon, J.H., Koller, L.D., and Kerkvliet, N.I., 1979, Lead-cadmium interaction—Effects on viral-induced mortality and tissue residues in mice: Archives of Environmental Health, v. 34, no. 6, p. 469–475. [Also available at https://doi.org/10.1080/00039896.1979.12088659.]
Fahim, M.S., and Khare, N.K., 1980, Effects of subtoxic levels of lead and cadmium on urogenital organs of male rats: Archives of Andrology, v. 4, no. 4, p. 357–362. [Also available at https://doi.org/10.3109/01485018008986982.]
Fei, D., Zhao, H., Wang, Y., Liu, J., Mu, M., Guo, M., Yang, X., and Xing, M., 2019, The disturbance of autophagy and apoptosis in the gizzard caused by copper and/or arsenic are related to mitochondrial kinetics: Chemosphere, v. 231, p. 1–9. [Also available at https://doi.org/10.1016/j.chemosphere.2019.05.101.]
Finelli, V.N., Klauder, D.S., Karaffa, M.A., and Petering, H.G., 1975, Interaction of zinc and lead on δ-aminolevulinate dehydratase: Biochemical and Biophysical Research Communications, v. 65, no. 1, p. 303–311. [Also available at https://doi.org/10.1016/S0006-291X(75)80093-3.]
Flora, S.J.S., Jain, V.K., Behari, J.R., and Tandon, S.K., 1982, Protective role of trace metals in lead intoxication: Toxicology Letters, v. 13, no. 1-2, p. 51–56. [Also available at https://doi.org/10.1016/0378-4274(82)90138-2.]
Flora, S.J.S., Kumar, D., and Gupta, S.D., 1991, Interaction of zinc, methionine or their combination with lead at gastrointestinal or post-absorptive level in rats: Pharmacology & Toxicology, v. 68, no. 1, p. 3–7. [Also available at https://doi.org/10.1111/j.1600-0773.1991.tb01199.x.]
Fowler, B.A., and Mahaffey, K.R., 1978, Interactions among lead, cadmium, and arsenic in relation to porphyrin excretion patterns: Environmental Health Perspectives, v. 25, p. 87–90. [Also available at https://doi.org/10.1289/ehp.782587.]
Franson, J.C., and Pain, D.J., 2011, Lead in Birds, in Beyer, W.N., and Meador, J.P., eds., Environmental contaminants in biota—Interpreting tissue concentrations: Boca Raton, Fla., CRC Press, p. 563–593. [Also available at https://doi.org/10.1201/b10598-17.]
French, A.D., Conway, W.C., Cañas-Carrell, J.E., and Klein, D.M., 2017, Exposure, effects and absorption of lead in American woodcock (Scolopax minor)—A review: Bulletin of Environmental Contamination and Toxicology, v. 99, no. 3, p. 287–296. [Also available at https://doi.org/10.1007/s00128-017-2137-z.]
Gad, S.C., 2014, Cobalt, in Wexler, P., ed., Encyclopedia of Toxicology (3rd ed.): Cambridge, Mass., Academic Press, p. 996–998. [Also available at https://doi.org/10.1016/B978-0-12-386454-3.00832-0.]
García-Fernández, A.J., Sánchez-García, J.A., Jiménez-Montalbán, P., and Luna, A., 1995, Lead and cadmium in wild birds in southeastern Spain: Environmental Toxicology and Chemistry, v. 14, no. 12, p. 2049–2058. [Also available at https://doi.org/10.1002/etc.5620141207.]
Gou, Z., Fan, Q., Li, L., Wang, Y., Lin, X., Cui, X., Ye, J., Ding, F., Cheng, Z., Abouelezz, K., and Jiang, S., 2021, High dietary copper induces oxidative stress and leads to decreased egg quality and reproductive performance of Chinese Yellow broiler breeder hens: Poultry Science, v. 100, no. 3, p. 100779. [Also available at https://doi.org/10.1016/j.psj.2020.10.033.]
Hutton, M., 1983, The effects of environmental lead exposure and in vitro zinc on tissue δ-aminolevulinic acid dehydratase in urban pigeons: Comparative Biochemistry and Physiology Part C—Comparative Pharmacology, v. 74, no. 2, p. 441–446. [Also available at https://doi.org/10.1016/0742-8413(83)90129-9.]
Konar, S.K., and Mullick, S., 1993, Problems of safe disposal of petroleum products, detergents, heavy metals and pesticides to protect aquatic life: Science of the Total Environment, v. 134, suppl. 2, p. 989–1000. [Also available at https://doi.org/10.1016/S0048-9697(05)80105-6.]
Koo, S.I., Fullmer, C.S., and Wasserman, R.H., 1978, Intestinal absorption and retention of 109Cd—Effects of cholecalciferol, calcium status, and other variables: The Journal of Nutrition, v. 108, no. 11, p. 1812–1822. [Also available at https://doi.org/10.1093/jn/108.11.1812.]
Kopp, S.J., Bárány, M., Erlanger, M., Perry, E.F., and Perry, H.M., Jr., 1980a, The influence of chronic low-level cadmium and/or lead feeding on myocardial contractility related to phosphorylation of cardiac myofibrillar proteins: Toxicology and Applied Pharmacology, v. 54, no. 1, p. 48–56.[ [Also available at https://doi.org/10.1016/0041-008X(80)90007-1.]
Kopp, S.J., Glonek, T., Erlanger, M., Perry, E.F., Bárány, M., and Perry, H.M., Jr., 1980b, Altered metabolism and function of rat heart following chronic low level cadmium/lead feeding: Journal of Molecular and Cellular Cardiology, v. 12, no. 12, p. 1407–1425. [Also available at https://doi.org/10.1016/0022-2828(80)90125-X.]
Kraak, M.H.S., Toussaint, M., Lavy, D., and Davids, C., 1994, Short-term effects of metals on the filtration rate of the zebra mussel Dreissena polymorpha: Environmental Pollution, v. 84, no. 2, p. 139–143. [Also available at https://doi.org/10.1016/0269-7491(94)90096-5.]
Li, J.-L., Jiang, C.-Y., Li, S., and Xu, S.-W., 2013, Cadmium induced hepatotoxicity in chickens (Gallus domesticus) and ameliorative effect by selenium: Ecotoxicology and Environmental Safety, v. 96, p. 103–109. [Also available at https://doi.org/10.1016/j.ecoenv.2013.07.007.]
Ling, J.R., and Leach, R.M., Jr., 1979, Studies on nickel metabolism—Interaction with other mineral elements: Poultry Science, v. 58, no. 3, p. 591–596. [Also available at https://doi.org/10.3382/ps.0580591.]
Lock, J.W., Thompson, D.R., Furness, R.W., and Bartle, J.A., 1992, Metal concentrations in seabirds of the New Zealand region: Environmental Pollution, v. 75, no. 3, p. 289–300. [Also available at https://doi.org/10.1016/0269-7491(92)90129-X.]
Mahaffey, K.R., and Fowler, B.A., 1977, Effects of concurrent administration of lead, cadmium, and arsenic in the rat: Environmental Health Perspectives, v. 19, p. 165–171. [Also available at https://doi.org/10.1289/ehp.7719165.]
Malhotra, K.M., Shukla, G.S., and Chandra, S.V., 1982, Neurochemical changes in rats coexposed to lead and copper: Archives of Toxicology, v. 49, no. 3-4, p. 331–336. [Also available at https://doi.org/10.1007/BF00347881.]
Mariakulandai, A., and McGinnis, J., 1953, The vitamin B12 requirement for hatchability of chicken eggs: Poultry Science, v. 32, no. 1, p. 3–7. [Also available at https://doi.org/10.3382/ps.0320003.]
Marlowe, M., Stellern, J., Errera, J., and Moon, C., 1985, Main and interaction effects of metal pollutants on visual-motor performance: Archives of Environmental Health, v. 40, no. 4, p. 221–225. [Also available at https://doi.org/10.1080/00039896.1985.10545922.]
Ming, H., He, W.-X., Lamb, D.T., Megharaj, M., and Naidu, R., 2012, Bioavailability of lead in contaminated soil depends on the nature of bioreceptor: Ecotoxicology and Environmental Safety, v. 78, p. 344–350. [Also available at https://doi.org/10.1016/j.ecoenv.2011.11.045.]
Mu, M., Zhao, H., Wang, Y., Liu, J., Fei, D., and Xing, M., 2019, Arsenic trioxide or/and copper sulfate co-exposure induce glandular stomach of chicken injury via destruction of the mitochondrial dynamics and activation of apoptosis as well as autophagy: Ecotoxicology and Environmental Safety, v. 185, p. 109678. [Also available at https://doi.org/10.1016/j.ecoenv.2019.109678.]
Nation, J.R., Grover, C.A., Bratton, G.R., and Salinas, J.A., 1990, Behavioral antagonism between lead and cadmium: Neurotoxicology and Teratology, v. 12, no. 2, p. 99–104. [Also available at https://doi.org/10.1016/0892-0362(90)90119-W.]
Negilski, D.S., Ahsanullah, M., and Mobley, M.C., 1981, Toxicity of zinc, cadmium and copper to the shrimp Callianassa australiensis—II. Effects of paired and triad combinations of metals: Marine Biology, v. 64, no. 3, p. 305–309, accessed August 2020 at https://doi.org/10.1007/BF00393631.]
Noerpel, M., Pribil, M., Rutherford, D., Law, P., Bradham, K., Nelson, C., Weber, R., Gunn, G., and Scheckel, K., 2020, Lead speciation, bioaccessibility and source attribution in Missouri’s Big River watershed: Applied Geochemistry, v. 123, p. 104757. [Also available at https://doi.org/10.1016/j.apgeochem.2020.104757.]
Osofsky, A., Jowett, P.L.H., Hosgood, G., and Tully, T.N., 2001, Determination of normal blood concentrations of lead, zinc, copper, and iron in Hispaniolan Amazon parrots (Amazona ventralis): Journal of Avian Medicine and Surgery, v. 15, no. 1, p. 31–36, accessed August 2020 at https://doi.org/10.1647/1082-6742(2001)015[0031:DONBCO]2.0.CO;2.]
Outridge, P.M., and Scheuhammer, A.M., 1993, Bioaccumulation and toxicology of nickel—Implications for wild mammals and birds: Environmental Reviews, v. 1, no. 2, p. 172–197, accessed August 2020 at https://doi.org/10.1139/a93-013.]
Park, S.Y., Birkhold, S.G., Kubena, L.F., Nisbet, D.J., and Ricke, S.C., 2004, Review on the role of dietary zinc in poultry nutrition, immunity, and reproduction: Biological Trace Element Research, v. 101, no. 2, p. 147–163. [Also available at https://doi.org/10.1385/BTER:101:2:147.]
Paternain, J.L., Domingo, J.L., and Corbella, J., 1988, Developmental toxicity of cobalt in the rat: Journal of Toxicology and Environmental Health, v. 24, no. 2, p. 193–200. [Also available at https://doi.org/10.1080/15287398809531153.]
Pedigo, N.G., and Vernon, M.W., 1993, Embryonic losses after 10-week administration of cobalt to male mice: Reproductive Toxicology (Elmsford, N.Y.), v. 7, no. 2, p. 111–116. [Also available at https://doi.org/10.1016/0890-6238(93)90244-2.]
Quenea, K., Lamy, I., Winterton, P., Bermond, A., and Dumat, C., 2009, Interactions between metals and soil organic matter in various particle size fractions of soil contaminated with waste water: Geoderma, v. 149, no. 3-4, p. 217–223. [Also available at https://doi.org/10.1016/j.geoderma.2008.11.037.]
Roels, H.A., Buchet, J.P., Bernard, A., Hubermont, G., Lauwerys, R.R., and Masson, P., 1978, Investigations of factors influencing exposure and response to lead, mercury, and cadmium in man and in animals: Environmental Health Perspectives, v. 25, p. 91–96. [Also available at https://doi.org/10.1289/ehp.782591.]
Scheuhammer, A.M., 1987, The chronic toxicity of aluminum, cadmium, mercury, and lead in birds—A review: Environmental Pollution, v. 46, no. 4, p. 263–295. [Also available at https://doi.org/10.1016/0269-7491(87)90173-4.]
Sileo, L., Nelson Beyer, W., and Mateo, R., 2003, Pancreatitis in wild zinc-poisoned waterfowl: Avian Pathology, v. 32, no. 6, p. 655–660. [Also available at https://doi.org/10.1080/03079450310001636246.]
Skinner, J.L., Quisenberry, J.H., and Couch, J.R., 1951, High efficiency and APF concentrates in the ration of the laying fowl: Poultry Science, v. 30, no. 3, p. 319–324. [Also available at https://doi.org/10.3382/ps.0300319.]
Stewart, F.M., Thompson, D.R., Furness, R.W., and Harrison, N., 1994, Seasonal variation in heavy metal levels in tissues of common guillemots, Uria aalge from northwest Scotland: Archives of Environmental Contamination and Toxicology, v. 27, no. 2, p. 168–175. [Also available at https://doi.org/10.1007/BF00214259.]
Sun, X., Li, J., Zhao, H., Wang, Y., Liu, J., Shao, Y., Xue, Y., and Xing, M., 2018, Synergistic effect of copper and arsenic upon oxidative stress, inflammation and autophagy alterations in brain tissues of Gallus gallus: Journal of Inorganic Biochemistry, v. 178, p. 54–62. [Also available at https://doi.org/10.1016/j.jinorgbio.2017.10.006.]
Thawley, D.G., Willoughby, R.A., McSherry, B.J., MacLeod, G.K., MacKay, K.H., and Mitchell, W.R., 1977, Toxic interactions among Pb, Zn, and Cd with varying levels of dietary Ca and vitamin D—Hematological system: Environmental Research, v. 14, no. 3, p. 463–475. [Also available at https://doi.org/10.1016/0013-9351(77)90053-6.]
Thompson, J., Jones, D.D., and Beasley, W.H., 1977, The effect of metal ions on the activity of δ-aminolevulinic acid dehydratase: Occupational and Environmental Medicine, v. 34, no. 1, p. 32–36, accessed June 2022 at https://doi.org/10.1136/oem.34.1.32.]
U.S. Environmental Protection Agency [EPA], 2005a, Ecological soil screening levels for cadmium [Interim final]: U.S. Environmental Protection Agency Office of Solid Waste and Emergency Response, OSWER Directive 9285.7–65, accessed August 2020 at https://www.epa.gov/sites/default/files/2015-09/documents/eco-ssl_cadmium.pdf.
U.S. Environmental Protection Agency [EPA], 2005b, Ecological soil screening levels for cobalt [Interim final]: U.S. Environmental Protection Agency Office of Solid Waste and Emergency Response, OSWER Directive 9285.7–67, accessed August 2020 at https://www.epa.gov/sites/default/files/2015-09/documents/eco-ssl_cobalt_.pdf.
U.S. Environmental Protection Agency [EPA], 2006a, Ecological risk assessment, Big River Mine tailings site, St. Francois County, Missouri, July 2006: U.S. Environmental Protection Agency Region 7 and Black and Veatch Special Projects Corps, accessed August 2020 at https://semspub.epa.gov/work/07/30285128.pdf.
U.S. Environmental Protection Agency [EPA], 2006b, Ecological soil screening levels for copper [Interim final]: U.S. Environmental Protection Agency Office of Solid Waste and Emergency Response, OSWER Directive 9285.7–68, revised February 2007, accessed August 2020 at https://www.epa.gov/sites/default/files/2015-09/documents/eco-ssl_copper.pdf.
U.S. Environmental Protection Agency [EPA], 2007, Ecological soil screening levels for nickel [Interim final]: U.S. Environmental Protection Agency Office of Solid Waste and Emergency Response, OSWER Directive 9285.7–76, accessed August 2020 at https://www.epa.gov/sites/default/files/2015-09/documents/eco-ssl_nickel.pdf.
van der Merwe, D., Carpenter, J.W., Nietfeld, J.C., and Miesner, J.F., 2011, Adverse health effects in Canada geese (Branta canadensis) associated with waste from zinc and lead mines in the Tri-State Mining District (Kansas, Oklahoma, and Missouri, USA): Journal of Wildlife Diseases, v. 47, no. 3, p. 650–660. [Also available at https://doi.org/10.7589/0090-3558-47.3.650.]
Voors, A.W., Johnson, W.D., and Shuman, M.S., 1982, Additive statistical effects of cadmium and lead on heart-related disease in a North Carolina autopsy series: Archives of Environmental Health, v. 37, no. 2, p. 98–102. [Also available at https://doi.org/10.1080/00039896.1982.10667544.]
Vranken, G., Tiré, C., and Heip, C., 1988, The toxicity of paired metal mixtures to the nematode Monhystera disjuncta (Bastian, 1865): Marine Environmental Research, v. 26, no. 3, p. 161–179. [Also available at https://doi.org/10.1016/0141-1136(88)90025-6.]
Wayland, M., and Scheuhammer, A.M., 2011, Cadmium in birds, in Beyer, W.N., and Meador, J.P., eds., Environmental contaminants in biota—Interpreting tissue concentrations: Boca Raton, Fla., CRC Press, p. 645–668. [Also available at https://doi.org/10.1201/b10598-21.]
Weber, J.S., Goyne, K.W., Luxton, T.P., and Thompson, A.L., 2015, Phosphate treatment of lead-contaminated soil—Effects on water quality, plant uptake, and lead speciation: Journal of Environmental Quality, v. 44, no. 4, p. 1127–1136. [Also available at https://doi.org/10.2134/jeq2014.10.0447.]
Wharton, H.M., Larsen, K.G., Sweeney, P.H., Harrison, E., Bradley, M., Davis, J.H., Rogers, R.K., Brown, W.J., Paarlberg, N.L., Evans, L.L., Mouat, M.M., and Clendenin, C.W., Jr., 1975, Guidebook to the geology and ore deposits of selected mines in the Viburnum Trend, Missouri: Missouri Department of Natural Resources Division of Research and Technical Information, Geological Survey, Report of Investigations no. 58, 60 p., accessed June 2022 at https://share.mo.gov/nr/mgs/MGSData/Books/Reports%20of%20Investigations/Guidebook%20to%20the%20Geology%20and%20Ore%20Deposits%20of%20Selected%20Mines% 20in%20the%20Viburnum%20Trend,%20Missouri/RI-058.pdf.
Wren, C.D., Nygård, T., and Steinnes, E., 1994, Willow ptarmigan (Lagopus lagopus) as a biomonitor of environmental metal levels in Norway: Environmental Pollution, v. 85, no. 3, p. 291–295. [Also available at https://doi.org/10.1016/0269-7491(94)90050-7.]
Yang, J., Mosby, D.E., Casteel, S.W., and Blanchar, R.W., 2002, In vitro lead bioaccessibility and phosphate leaching as affected by surface application of phosphoric acid in lead-contaminated soil: Archives of Environmental Contamination and Toxicology, v. 43, no. 4, p. 399–405, accessed August 2020 at https://doi.org/10.1007/s00244-002-1197-0.
Conversion Factors
International System of Units to U.S. customary units
Temperature in degrees Celsius (°C) may be converted to degrees Fahrenheit (°F) as follows:
°F = (1.8 × °C) + 32.
Datum
Coordinates are provided in decimal degrees.
Vertical and horizontal coordinate information is referenced to the World Geodetic System 84 coordinate system (WGS 84).
Abbreviations
>
greater than
≥
greater than or equal to
<
less than
±
plus or minus
Δ
change in
δALAD
δ-aminolevulinic acid dehydratase
µm
micrometer
µM
micromole per liter
µL
microliter
8-OH-dG
8-hydroxy-2'-deoxyguanosine
°C
degree Celsius
ALA
aminolevulinic acid substrate
ANOVA
analysis of variance
ATSDR
Agency for Toxic Substances and Disease Registry
BERA
baseline ecological risk assessment
BloodPb
lead concentration in blood
CCVS
continuing calibration verification standard
cm
centimeter
CRM
certified reference material
CV
coefficient of variation
df
degree of freedom
DIC
deviance information criteria
DNA
deoxyribonucleic acid
dw
dry-weight basis
Eco-SSL
Ecological Soil Screening Levels
EPA
U.S. Environmental Protection Agency
g
gram
GLM
generalized linear mixed
GPS
global positioning system
GSH
reduced glutathione
GSSG
oxidized glutathione
ICP–MS
inductively coupled plasma-mass spectrometry
ICVS
initial calibration verification standard
IDW
inverse-distance-weighted
LOAEL
lowest observable adverse effects level
LOD
limit of detection
log
logarithmic (common, base 10)
LOQ
limit of quantification
m
meter
M
molar
MCMC
Markov chain Monte Carlo
mg
milligram
mg/kg
milligram per kilogram
mg/kg BW/d
milligram per kilogram per body weight per day
mg/mL
milligram per milliliter
mM
millimolar
n
number of replicates or individuals
NBF
neutral buffered formalin
ND
not detected
ng/mL
nanogram per milliliter
p
probability value
PBS
phosphate-buffered saline
PBSH
protein bound sulfhydryl
ppm
part per million
pXRF
portable X-ray fluorescence spectrometer
QC
quality control
R2
coefficient of determination
ROS
reactive oxygen species
SD
standard deviation
SoilPb
lead concentration in soil
TBARS
thiobarbituric acid reactive substances
tGSH
total glutathione
TRV
toxicity reference value
TSH
total sulfhydryl
USFWS
U.S. Fish and Wildlife Service
USGS
U.S. Geological Survey
ww
wet weight
For more information about this publication, contact:
Director, USGS Columbia Environmental Research Center
4200 New Haven Road
Columbia, MO 65201
573–875–5399
For additional information, visit: https://www.usgs.gov/centers/cerc
Publishing support provided by the
Rolla Publishing Service Center
Disclaimers
Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Although this information product, for the most part, is in the public domain, it also may contain copyrighted materials as noted in the text. Permission to reproduce copyrighted items must be secured from the copyright owner.
Suggested Citation
Brasso, R., Cleveland, D., Thompson, F.R., III, Mosby, D.E., Hixson, K., Roach, M., Rattner, B.A., Karouna-Renier, N.K., and Lankton, J.S., 2023, Effects of lead exposure on birds breeding in the Southeast Missouri Lead Mining District: U.S. Geological Survey Scientific Investigations Report 2023–5032, 127 p., https://doi.org/10.3133/sir20235032.
ISSN: 2328-0328 (online)
Study Area
| Publication type | Report |
|---|---|
| Publication Subtype | USGS Numbered Series |
| Title | Effects of lead exposure on birds breeding in the Southeast Missouri Lead Mining District |
| Series title | Scientific Investigations Report |
| Series number | 2023-5032 |
| DOI | 10.3133/sir20235032 |
| Publication Date | August 11, 2023 |
| Year Published | 2023 |
| Language | English |
| Publisher | U.S. Geological Survey |
| Publisher location | Reston, VA |
| Contributing office(s) | Columbia Environmental Research Center, Patuxent Wildlife Research Center, Eastern Ecological Science Center |
| Description | Report: xi, 127 p.; Data Release |
| Country | United States |
| State | Missouri |
| Online Only (Y/N) | Y |


