Evaluation of Passive Samplers for Cyanotoxin Detection by Immunoassay and Chromatographic-Mass Spectrometry
Links
- Document: Report (3.86 MB pdf) , HTML , XML
- Dataset: USGS National Water Information System database - USGS water data for the Nation
- Data Release: USGS data release - Cyanotoxin concentrations in extracts from solid phase adsorption toxin tracking (SPATT) and diffusive gradients in thin-films (DGT) samplers in Owasco Lake, Seneca Lake, and Skaneateles Lake, Finger Lakes Region, New York, 2019
- Download citation as: RIS | Dublin Core
Acknowledgments
This study was supported as part of an advanced monitoring pilot study of cyanobacterial harmful algal blooms, funded by the New York State Department of Environmental Conservation and by the U.S. Geological Survey. The authors acknowledge the participation of State University of New York College of Environmental Science and Forestry students in sample processing.
The authors also acknowledge the field and laboratory assistance of S. Gifford, K. Finkelstein, and many other dedicated U.S. Geological Survey staff members.
Abstract
Harmful algal blooms, particularly cyanobacterial harmful algal blooms, threaten aquatic ecosystems, drinking water supplies, and recreational resources. In 2019, the U.S. Geological Survey, in collaboration with the New York State Department of Environmental Conservation, deployed solid phase adsorption toxin tracking (SPATT) samplers in Seneca Lake, Owasco Lake, and Skaneateles Lake to monitor the cyanotoxins microcystins, cylindrospermopsins, anatoxins, and saxitoxins. SPATT samplers can passively adsorb dissolved cyanotoxins over time, providing time-integrated data capable of detecting low concentrations of cyanotoxins that traditional discrete sampling may miss. SPATT samples were analyzed using enzyme-linked immunosorbent assay (ELISA), liquid chromatography with mass spectrometry (LC–MS), and with tandem mass spectrometry (LC–MS/MS). The effects of ELISA-required preservative on measurements by mass spectrometry methods were also evaluated.
SPATT samplers consistently detected microcystins and anatoxins more frequently than concurrent discrete sampling. ELISA results often showed higher cyanotoxin concentrations than LC–MS/MS, likely due to interference from dissolved organic matter and the ability of ELISA to detect a broader range of congeners. The addition of preservative influenced results for some analytes, particularly microcystins, which showed higher concentrations in preserved samples. Limitations in ELISA methods for cylindrospermopsins and saxitoxins were identified, potentially related to cross-reactivity, low sensitivity, or other matrix interferences. This study demonstrates the utility of SPATT samplers in capturing cyanotoxin variability, especially in environments with low cyanotoxin levels or ephemeral blooms. Further research could help improve the reliability of ELISA and other analytical methods in freshwater ecosystems.
Introduction
Harmful algal blooms can negatively affect aquatic ecosystems, through reductions in dissolved oxygen and light availability, and the economy, through the loss of tourism revenue, fishery closures, and reduced property values (Havens, 2008; Dodds and others, 2009). One phenomenon of particular interest is cyanobacterial harmful algal blooms (cyanoHABs), which can produce a variety of toxins (called cyanotoxins), as well as compounds that affect the taste and smell of water. These cyanotoxins and taste-and-odor compounds are of concern in waterbodies used to supply drinking water or for recreation (Boyer, 2007; Graham and others, 2008). Cyanotoxins have caused illness in humans and illness and death in animals throughout the United States (Hudnell, 2008; Trevino-Garrison and others, 2015). Early detection and preventative management are increasingly important because cyanoHAB occurrences have increased globally over the past several decades (O’Neil and others, 2012; Trevino-Garrison and others, 2015; Taranu and others, 2015; Favot and others, 2023; Gorney and others, 2023). The Finger Lakes region of central New York has likewise had an increase in cyanoHABs in recent decades. In 2017, all 11 Finger Lakes had open water, shoreline, or both types of cyanoHABs (Boyer, 2007; New York State Department of Environmental Conservation [NYSDEC], 2018, 2020).
The U.S. Geological Survey (USGS), in collaboration with the NYSDEC, conducted a cyanoHAB advanced monitoring pilot study to improve the state of monitoring and understand cyanoHABs in the Finger Lakes. As part of this pilot study, a series of assessments were carried out between 2018 and 2020 to evaluate a range of traditional and innovative monitoring approaches and technologies. The objectives of the assessments were to inform future monitoring strategies and increase the understanding of factors related to cyanoHAB proliferation in New York State.
In 2019, the USGS used solid phase adsorption toxin tracking (SPATT) samplers across three low- to moderate-nutrient lakes that undergo seasonal stratification: Seneca Lake, Owasco Lake, and Skaneateles Lake (fig. 1). SPATT samplers are passive devices designed to adsorb dissolved cyanotoxins and characterize cyanotoxin occurrence over time (MacKenzie and others, 2004; Kudela, 2011; Wood and others, 2011; Howard and others, 2017; Kudela, 2017; Roué and others, 2018). The primary advantage of using SPATT samplers for cyanotoxin monitoring is their potential to provide concentrated measurements of cyanotoxins in the water column, which may enable the detection of ephemeral occurrences missed by discrete sampling approaches. However, due to the time integration, the concentrations of cyanotoxins measured from SPATTs cannot be easily related to ambient concentrations in the water because of variations in flow velocity and cyanotoxin concentration over the deployment period and other environmental factors (Kudela, 2011). Variations in flow velocity influence the rate of cyanotoxin adsorption onto the SPATT resin, with higher flow potentially increasing adsorption efficiency. Similarly, fluctuating cyanotoxin concentrations in the water during the deployment period affect the cumulative amount of cyanotoxin adsorbed. Additionally, SPATT samplers can only adsorb and concentrate dissolved cyanotoxins, whereas discrete samples can measure both dissolved and nondissolved (intracellular) cyanotoxins.
Despite their limitations, SPATT samplers can offer a more comprehensive understanding of cyanotoxin dynamics over time, compared with the snapshot obtained from traditional discrete sampling methods (Lane and others, 2010; Kudela, 2011, 2017). Whereas SPATT samplers are considered a semi-quantitative approach, their high sensitivity and inexpensive construction can make them a valuable supplement to standard monitoring practices (Lane and others, 2010; MacKenzie, 2010; Wood and others, 2011). In this study, SPATT samplers were deployed at multiple depths in each lake to evaluate their ability to provide information on cyanotoxins over time and depth, and to compare differences in results from samplers positioned near each other.
The goal of this study was to evaluate the ability of SPATT samplers to provide measurements of four classes of cyanotoxins—microcystins, cylindrospermopsins, anatoxins, and saxitoxins—and to assess the effect of an enzyme-linked immunosorbent assay (ELISA)-required preservative on measurements by mass spectrometry methods. This information will help refine best practices and improve the understanding of SPATT results, contributing to more effective application and interpretation of this monitoring approach for cyanoHABs. All data discussed in this report are available in a USGS data release (Stouder and others, 2024) or the National Water Information System (NWIS; USGS, 2016).
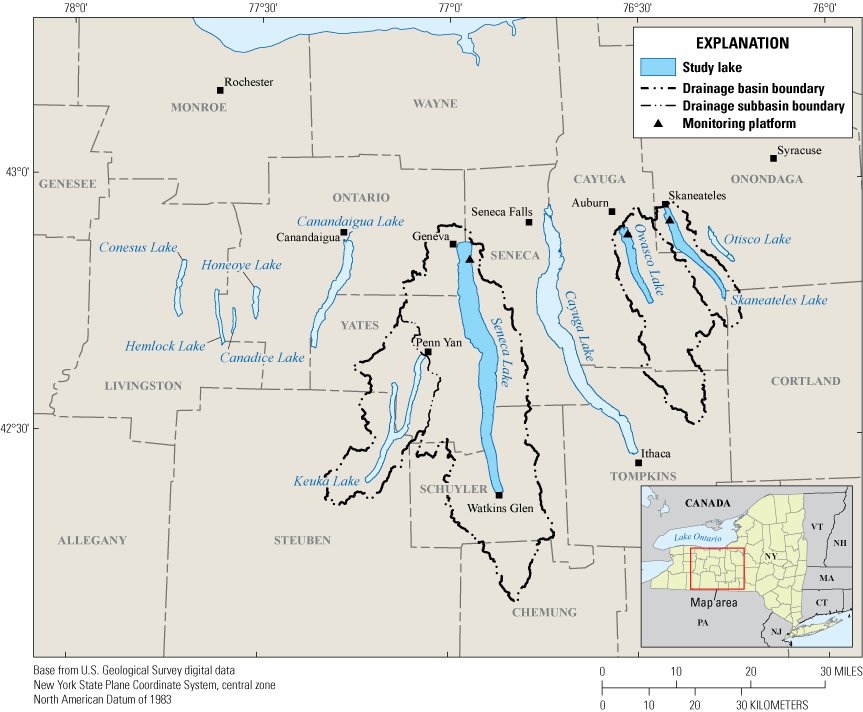
Map of the Finger Lakes region, New York, including the three study locations within Seneca Lake, Owasco Lake, and Skaneateles Lake.
Description of Study Area
The Finger Lakes are important recreational, drinking water, and economic resources for New York State (NYSDEC, 2019; Halfman and others, 2023). The Finger Lakes region encompasses 11 narrow north-south-oriented glacial lakes in western New York, south of Lake Ontario (not shown; fig. 1). About 8 percent of New York is in the Finger Lakes watershed, which covers about 12,000 square kilometers (km2) and all or part of 12 counties (Callinan, 2001). In recent decades, cyanoHAB frequency has increased in the Finger Lakes (NYSDEC, 2020). In 2017, all 11 Finger Lakes experienced open water or shoreline cyanoHABs, even those historically characterized by low concentrations of nutrients and chlorophyll a (NYSDEC, 2018). Previous studies on the Finger Lakes and other lakes in New York State have documented the spatiotemporal heterogeneity of cyanoHABs and cyanotoxins within and among lakes (NYSDEC, 2019; Smith and others, 2019; Prestigiacomo and others, 2023; Gorney and others 2023). Seneca Lake (USGS site 425027076564401), Owasco Lake (USGS site 425327076313601), and Skaneateles Lake (USGS site 425606076251601; for additional information about all sites, refer to U.S. Geological Survey, 2016) were chosen for this pilot study in part because they represent a continuum of trophic states, from oligotrophic (low nutrient concentrations) to mesotrophic (moderate nutrient concentrations; Callinan, 2001; NYSDEC, 2019).
Of the 11 Finger Lakes, Seneca Lake is the second largest in surface area (about 175 km2) and the deepest (maximum depth is about 200 meters [m]). Seneca Lake has a volume of about 15,500 million cubic meters and a drainage area of about 1,200 km2. Owasco Lake is the sixth largest Finger Lake in surface area (about 30 km2), has a maximum depth of about 50 m, and has a volume of about 780 million cubic meters. The Owasco Lake drainage area is about 470 km2. Skaneateles Lake is the fifth largest Finger Lake in surface area (about 40 km2), has a maximum depth of about 90 m, and has a volume of about 1,600 million cubic meters. The Skaneateles Lake drainage area is about 150 km2 (Callinan, 2001).
A substantial part of land use in the watersheds of all three lakes is agricultural—about 40 percent for Seneca Lake, about 55 percent for Owasco Lake, and about 40 percent for Skaneateles Lake. Data from 2018 indicate that Seneca and Owasco Lakes are mesotrophic (total phosphorus at Seneca and Owasco Lakes was 0.011 milligrams per liter [mg/L] and 0.008 mg/L, respectively, and total nitrogen was 0.551 mg/L and 0.948 mg/L, respectively), and that Skaneateles Lake is oligotrophic (total phosphorus and total nitrogen were 0.004 mg/L and 0.471 mg/L, respectively; NYSDEC, 2019).
SPATT Sampler Methods
SPATT samplers can be an effective way to detect and measure dissolved cyanotoxins in water (Kudela, 2011; Wood and others, 2011; Howard and others, 2017). SPATT samplers are inexpensive to make and generally involve simple protocols for deployment and retrieval (Kudela, 2011; Howard and others, 2018). However, SPATT samplers can be subject to matrix interference—that is, substances naturally present in water that can affect the accuracy of measurements taken by a water quality monitoring tool—including nonspecific adsorption of nontarget analytes, which can result in inaccurate cyanotoxin quantification (Sangolkar and others, 2006; He and others, 2016; Birbeck and others, 2019; Jaramillo and O’Shea, 2019).
The SPATT method involves construction, deployment, and subsequent retrieval of the samplers, followed by extraction and analysis for cyanotoxins in the laboratory (Howard and others, 2018). This study compared three analytical methods: ELISA, liquid chromatography with mass spectrometry (LC–MS), and liquid chromatography with tandem mass spectrometry (LC–MS/MS). ELISA provides a measure of the aggregate concentration of a specific class of cyanotoxins, such as microcystins or anatoxins, (Sangolkar and others, 2006; Gaget and others, 2017; Jaramillo and O’Shea, 2019). The aggregate concentration is independent from congeners; that is, molecular variations of cyanotoxins in the same class. Mass spectrometry methods (that is, LC–MS and LC–MS/MS) can offer greater specificity with detailed information about the presence of specific congeners. However, these two methods are limited to quantifying only those cyanotoxins that are included in the analytical protocol (Birbeck and others, 2019). The total cyanotoxin concentration determined by mass spectrometry represents a summation of the individual cyanotoxin congeners measured, in contrast to the aggregate measurement obtained with ELISA. LC–MS/MS can reduce interferences and offer greater specificity in complex samples than LC–MS but requires more sophisticated equipment, and its effectiveness is even more dependent on the availability of analytical standards (Ho and others, 2003). Mass spectrometry has higher start-up costs than ELISA because of the need for expensive instrumentation and highly trained operators, but the cost per sample is generally lower once the equipment has been obtained (Gaget and others, 2017). Because both methods have trade-offs on reliability, cost, and accuracy, comparisons of paired findings across methods are valuable (Gaget and others, 2017; Birbeck and others 2019).
SPATT Sampler Construction and Deployment
The SPATT samplers used in this study were constructed using modifications of methods described in Lane and others (2010). A 3-gram (g) portion of DIAION HP20 (Mitsubishi Chemical Group, Tokyo, Japan), a highly porous, cross-linked, styrene-divinylbenzene synthetic adsorbent resin, was used as the adsorbent material. HP20 is the most common resin used in SPATT sampler studies, and 3 g is the most common and most effective amount of resin per sampler (MacKenzie, 2010; Zendong and others, 2016; Roué and others, 2018). The HP20 resin beads were contained between two layers of 100-µm Nitex nylon mesh assembled on a 12.7-cm plastic embroidery hoop (fig. 2). Prior to deployment, the SPATT samplers were activated by soaking in 100-percent methanol for 24 hours, triple-rinsed with and stored in ultra-pure deionized water, and kept refrigerated. SPATT samplers were attached along a weighted steel cable that was deployed between mid-May and mid-November 2019, below an open-water monitoring platform (as described in Johnston and others, 2024) located near the northernmost end of each study lake. The SPATT samplers were located at near-surface, middle, and near-bottom depths; the same depths at which continuous water-quality sensor data and discrete water-quality samples were collected. (table 1; figs. 1 and 2; Johnston and others, 2024).
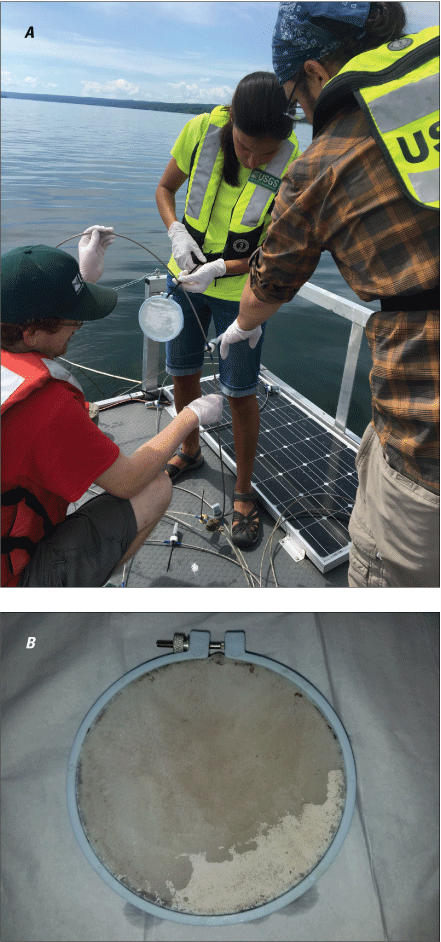
Photographs of solid phase adsorption toxin tracking (SPATT) samplers. A, Sampler retrieved from deployment. Photograph by U.S. Geological Survey. B, A sampler being prepared for deployment. Photograph by Jennifer Graham, U.S. Geological Survey.
SPATT Sampler Collection and Analysis
SPATT samplers were retrieved after collection periods lasting between 5 and 22 days, sealed in plastic bags, and kept cold on wet ice until being placed in a freezer later that day. The SPATT samplers were stored frozen for up to 6 months before being shipped on dry ice to the USGS Oregon Water Science Center in Portland for extraction and analysis by ELISA for four types of cyanotoxins (microcystins, cylindrospermopsins, anatoxins, saxitoxins). Cyanotoxins were extracted from the SPATT resin using a 50-percent solution of LC–MS-grade methanol and organic-free water (Howard and others, 2018). These extract samples were evaporated to dryness and reconstituted in a 2-percent methanol and organic-free water solution before analysis to achieve methanol concentrations less than 2.5 percent per the ELISA manufacturer’s limit for anatoxin-a (Carpenter and Wise, 2023; Gold Standard Diagnostics, 2024a–d).
A subset of comparison samples was chosen to represent the range of deployment conditions including the study lakes, deployment depths, and cyanotoxin concentrations as indicated by the ELISA results. These samples were sent to the State University of New York College of Environmental Science and Forestry and were analyzed by LC–MS for microcystins only, and by LC–MS/MS for microcystins, cylindrospermopsins, and anatoxins. No mass spectrometry analyses were performed for saxitoxins because of inconsistencies in published methods (for example, Li and Persson, 2021) with freshwater saxitoxin analogs. Because ELISA preservatives added to SPATT samples may interfere with mass spectrometry analyses, the subset of SPATT samples analyzed by mass spectrometry methods included 22 pairs of preserved and unpreserved samples to evaluate this potential effect.
SPATT Extract Samples Analyzed by ELISA
SPATT extract samples were thawed and split into two separate aliquots: one for neurotoxin (that is, anatoxins and saxitoxins) analyses, the other for protein synthesis inhibitor (that is, microcystins and cylindrospermopsins) analyses. The samples were analyzed using protocols outlined in the Gold Standard Diagnostics (Warminster, Pennsylvania) instruction manuals (Gold Standard Diagnostics, 2024a–d) for the following ABRAXIS test kits: microcystins and nodularins (product number 520011), anatoxin-a (product number 520060), cylindrospermopsins (product number 522011), and saxitoxins (product number 52255B). Extract samples with concentrations exceeding the upper limit of the ELISA calibration curves (microcystins [ADDA-specific, referring to moiety (4E,6E)-3-amino-9-methoxy-2,6,8-trimethyl-10-phenyldeca-4,6-dienoic acid]: 5.00 µg/L; anatoxins: 5.00 µg/L; cylindrospermopsins: 2.00 µg/L; and saxitoxins: 0.4 µg/L) were diluted and reanalyzed. The method detection limit (MDL) varied by cyanotoxin group (microcystins [ADDA-specific]: 0.15 µg/L; anatoxins: 0.15 µg/L; cylindrospermopsins: 0.05 µg/L; and saxitoxins: 0.02 µg/L) and was determined based on the lowest concentration nonzero standard found in each respective ELISA kit.
LC–MS and LC–MS/MS Analysis of SPATT Extract Samples
SPATT extract samples were analyzed for microcystins by LC–MS using a Waters Micromass ZQ 4000 mass detector for nodularin and 21 microcystin congeners (refer to table 2) as described in Boyer (2020). Extracted samples were analyzed for microcystins by LC–MS/MS using a Thermo Scientific Quantiva Altis triple quadrupole mass spectrometer according to the methods described in Birbeck and others (2019) for nodularin and 11 microcystin congeners (RR, YR, HtyR, dLR, LR, HilR, WR, LA, LY, LW, and LF; refer to table 2). The samples also were analyzed by LC–MS/MS for three cylindrospermopsins congeners (cylindrospermopsin, 7-epi-cylindrospermopsin, and 7-deoxycylindrospermopsin) and four anatoxins congeners (anatoxin-a, homoanatoxin-a, dihydroanatoxin-a, and dihydrohomoanatoxin-a; additional details regarding anatoxins methods are described in Barnard and others [2021]). The analysis utilized a modified version of U.S. Environmental Protection Agency (EPA) Method 545, adding multiple congeners along with a quantification ion and two confirmatory ions for each cyanotoxin (EPA, 2015).
Table 2.
List of analyzed microcystin congeners and their amino acid modifications and abbreviations.[Amino acids are abbreviated as follows: A, alanine; F, phenylalanine; L, leucine; R, arginine; W, tryptophan; Y, tyrosine]
For cyanotoxins analyzed by LC–MS or LC–MS/MS, the MDLs (available in Stouder and others [2024]) were determined on the day of analysis, based on sample volume, extract volume, injection volume, and instrument reporting limits at the time (Boyer, 2020). For microcystins, MDLs were reported as microcystin-LR equivalents, and total concentrations were calculated as the sum of all congeners. For cylindrospermopsins and anatoxins, detections included an additional data quality check not part of the standard EPA 545 method. This check involved using one quantification ion and two confirmatory ions, with detections categorized as confirmed, possible, or suspect based on the presence of the parent and confirmation ions in the correct ratio (Conklin and others, 2020).
Discrete Sample Methods
As part of the advanced monitoring pilot study, discrete water-quality samples were collected every 2 weeks. These samples were analyzed for biological and physicochemical variables, including immunoassay (ELISA) and chromatographic (that is, mass spectrometry; LC–MS and LC–MS/MS) quantification of total cyanotoxin concentrations in whole water. Discrete samples that coincided with SPATT recoveries were used to aid in interpretation of the SPATT results.
Discrete Sample Collection and Analysis
Environmental water was collected using an 8-liter (L), polyvinyl chloride (PVC) vertical Van Dorn sampler (USGS, variously dated; Graham and others, 2008) from the same depths at which SPATT samplers were deployed. The collected water was transferred into an 8-L fluoropolymer churn splitter, which allows for homogenization and the subsampling required for different analyses (USGS, variously dated).
Analysis of Discrete Samples by ELISA
Discrete unfiltered water samples for total cyanotoxin analyses were collected in 250-milliliter (mL) high-density polyethylene (HDPE) bottles, kept cold on wet ice until being placed in a freezer later that day, and stored frozen. Prior to analysis, samples were thawed and split into two separate aliquots, one for microcystins and cylindrospermopsins analyses, the other for anatoxins and saxitoxins analyses. The aliquots for anatoxins and saxitoxins analyses were preserved using a preservative supplied in the ELISA kits. The samples were analyzed using Gold Standard Diagnostics (2024a–d) ABRAXIS ELISA kits as described above for SPATT extract samples. Samples were processed through three freeze/thaw cycles to lyse cyanobacterial cells. Lysates were then filtered with 0.45-µm glass-fiber filters and analyzed for all four types of cyanotoxins at the USGS in Troy, New York. Whereas the MDLs of these are the same as those described for SPATT extract samples, the minimum reporting level for each cyanotoxin determined at the USGS in Troy was defined as twice the detection limit (microcystins: 0.30 µg/L; ADDA-specific, anatoxins: 0.30 µg/L; cylindrospermopsins: 0.10 µg/L; and saxitoxins: 0.04 µg/L) in accordance with EPA Method 546 (EPA, 2016). ELISA cyanotoxin results are available in NWIS (USGS, 2016).
LC–MS and LC–MS/MS Analysis of Discrete Samples
About half the volume of the thawed 250-mL unfiltered water samples used for ELISA discussed above was separated into aliquots of 100–150 mL and sent to State University of New York College of Environmental Science and Forestry on wet ice. These unfiltered water samples were centrifuged, and supernatants were filtered using methods comparable to those described in Barnard and others (2021) prior to analysis. Samples were analyzed for microcystins by LC–MS, and cylindrospermopsins and anatoxins by LC–MS/MS using methods described above for SPATT extract samples. LC–MS and LC–MS/MS cyanotoxin results are available in NWIS (USGS, 2016).
Sample Quality Assurance and Control
Quality control samples were collected for quality assurance, accounting for about 20 percent of all SPATT samplers deployed. Sequential unfiltered water replicate samples were used to evaluate the variability resulting from environmental factors and sample collection and processing. Replicate pairs of SPATT samplers were deployed side by side at all lakes and all depths. The coefficient of variation was used to compare replicate results (Zar, 1999); however, the coefficient of variation was not calculated for any replicate pairs where one, or both, of the replicate concentrations were below the MDL and (or) flagged as “estimated” due to being above the calibration curve and not diluted for further analysis. Additionally, the coefficients of variation of LC–MS and LC–MS/MS results were not calculated for diluted samples (that is, dilution resulting from preceding ELISA procedure). These factors can introduce additional variability and potential errors into the measurements, the implications of which were considered outside the scope of this study. A total of 22 replicate pairs of SPATT samplers were deployed over the course of the study, and 15 (about 68 percent) were recovered with both samplers intact. For samples with no detectable cyanotoxin concentrations, the same finding held for its replicate, except for one unpreserved microcystins pair analyzed by LC–MS, where microcystins were detected at low concentrations in one replicate but not the other. The median and mean coefficients of variation between replicate pairs for all methods and cyanotoxins were less than or equal to 25 and 35 percent, respectively (table 3). The highest coefficients of variation were observed in preserved samples analyzed for microcystins by LC–MS and LC–MS/MS. The maximum coefficients of variation of those samples were more than double those of unpreserved samples. This suggests that the preservative supplied in the ELISA kits may have affected the analysis by mass spectrometry methods, or it may have been an artifact of the smaller number of unpreserved replicate pairs. Sample results that were below the MDL or labeled as “estimated,” as well as LC–MS and LC–MS/MS results from diluted samples, were also excluded from the method comparison results of this report.
Table 3.
Coefficient of variation between replicate pairs of solid phase adsorption toxin tracking extract samples for each cyanotoxin by each analysis method, including preserved and unpreserved samples.[Data from Stouder and others (2024); National Water Information System (USGS, 2016). ELISA, enzyme-linked immunosorbent assay; LC–MS, liquid chromatography with mass spectrometry; LC–MS/MS, liquid chromatography with tandem mass spectrometry; —, not applicable]
Two blank extract samples were taken from two SPATT samplers not deployed in the field. The blank SPATT samplers were activated, stored, extracted, and analyzed in the same manner as the deployed SPATT samplers. The blank samples were analyzed by ELISA, and one of them was also analyzed by LC–MS/MS. Analyses by ELISA did not detect microcystins or anatoxins, but cylindrospermopsins were detected at or near the ELISA MDL in both blank extract samples. Saxitoxins were detected above the ELISA MDL (about 0.03 µg/L) in one of the blank extract samples. The single LC–MS/MS analysis included a false positive for microcystins (at about 3.0 µg/L), but no other cyanotoxins were detected. This false positive was attributed to the presence of peaks in nonspecific channels during the LC–MS/MS detection process. Such peaks can arise from instrument noise or interference from other compounds, leading to misidentification by the detection software. Given the stringent laboratory quality control measures in place for this study, as described in Boyer (2020) and Carpenter and Wise (2023), this single occurrence was considered an anomaly that is not indicative of the overall reliability of the study findings.
Analytical Challenges for Cylindrospermopsins and Saxitoxins Detections
Cylindrospermopsins were detected at less than 2.0 µg/L across all SPATT extract samples analyzed by ELISA, with no confirmatory detections by LC–MS/MS. Although discussion of cyanotoxin synthetase gene analysis and broader molecular results is outside the scope of this report, the molecular analysis of discrete water samples collected during this study did not indicate the presence of cylindrospermopsins synthetase genes (data available in NWIS [USGS, 2016]). Synthetase genes are responsible for producing the enzymes that synthesize specific cyanotoxins, and their absence suggests that cyanobacteria with the genetic capacity to produce cylindrospermopsins were not present in the analyzed samples. This finding, coupled with a decade of State monitoring indicating no cylindrospermopsins detections (NYSDEC, 2024), casts doubt on the accuracy of the ELISA results for cylindrospermopsins. False positives in these results may be attributed to two apparent factors: (1) the yellow-brown coloration observed in SPATT extract samples, indicative of dissolved organic matter, which is known to cause ELISA interference (Nunes and others, 1998; Huang and Sedlak, 2001; Hanselman and others, 2004, Silva and others, 2014); and (2) the detection of cylindrospermopsins of about 0.05 µg/L in blank SPATT extract samples, which indicates one or multiple errors in the analysis method.
Saxitoxins were detected at less than about 0.40 µg/L across all SPATT extract samples analyzed by ELISA. However, these samples were not confirmed with LC–MS/MS analysis because of inconsistencies in published methods. Although low concentrations of saxitoxins have been historically documented in cyanoHAB studies of New York State lakes, the consistent low-level detection across all samples and dates of collection suggests possible interference (presumably related to dissolved organic matter) in the ELISA results, especially considering that saxitoxins occurrence is typically seasonal (Smith and others, 2019, 2020). In contrast, more variation in the sample concentrations would be expected over time if cyanoHABs created saxitoxins in the lakes. Previous studies using SPATT samplers for saxitoxins detection have produced inconsistent results (Lane and others, 2010; Hattenrath-Lehmann and others, 2018), highlighting a need for further methodological refinement. Furthermore, most saxitoxin detections within New York State have been caused by cross reactivity of the analytical method to a different congener of the paralytic shellfish toxins called the Lyngbya wollei toxins (Carmichael and others, 1997); for most detections, additional testing has shown that the parent compound, saxitoxin, is generally low or absent (Foss and others, 2012; Smith and others, 2019, Smith and Boyer, 2024). The saxitoxin-ELISA used to analyze samples in this study shows some cross-reactivity with these and other toxins (Gold Standard Diagnostics, 2024d), which means that the saxitoxin detections in this study may be false positives. Because of these analytical challenges and the strong indicators of interference potentially causing positive bias and (or) false positives in the results, the ELISA detections of cylindrospermopsins and saxitoxins in this study are considered unreliable and are not discussed further in this report.
Results of Cyanotoxin Analyses
From June through November 2019, 110 SPATT samplers were deployed for durations between 5 and 22 days, averaging 14 days across all recovered samplers. About 86 percent (95 samplers) were recovered intact and processed for analysis (tables 4 and 5). Some SPATT samplers were not usable because of damage (loss of some mesh and resin) or were lost because of turbulent conditions in the lake, particularly at the near-surface depths. Of the 95 samplers recovered intact, 15 were replicate samplers discussed in the previous section and are not included in the following results discussion. Additionally, seven samplers submitted for inter-laboratory comparison were not considered because results were not available in time for this report; these data are available in Stouder and others (2024).
Table 4.
The number of solid phase adsorption toxin tracking samplers deployed, recovered, and analyzed, by lake and deployment depth.[Data from Stouder and others (2024); National Water Information System (USGS, 2016). m, meter; —, not applicable]
Table 5.
Number of samples analyzed for microcystins and anatoxins by each method, percent with detections, and range in detection limits[Data from Stouder and others (2024); National Water Information System (USGS, 2016). MDL, minimum detection limit; µg/L, microgram per liter; LC–MS, liquid chromatography with mass spectrometry; LC–MS/MS, liquid chromatography with tandem mass spectrometry; ELISA, enzyme-linked immunosorbent assay]
Linear regressions and quantitative analyses were used to assess the relations between cyanotoxin concentrations and two variables: laboratory analysis method and preservation. Specifically, the regressions between analytical methods or preservation status and cyanotoxin concentrations were examined using ordinary least squares analysis (Helsel and others, 2020). All data were compiled using R statistical software (R Core Team, 2022).
One hundred percent (71 out of 71) of the microcystins concentrations and 94 percent (67 out of 71) of the anatoxins concentrations in the SPATT extract samples exceeded the upper limit of the ELISA calibration curves (5.00 µg/L for both assays), requiring further dilutions and reanalysis to achieve results in a quantifiable range. ELISA cyanotoxin concentrations for the SPATT samples in this study are available in Stouder and others (2024).
Results of Microcystins Analyses
Microcystins were detected in all the samples analyzed by ELISA and LC–MS/MS and in most samples analyzed by LC–MS (table 5). The extensive detection of microcystins by all three analytical methods shows that this cyanotoxin was common in the study lakes during the study period. Of all the SPATT samplers analyzed, 23 measurements by ELISA, 45 by LC–MS, and 71 by LC–MS/MS were considered satisfactory for consideration in these results (table 6). Overall, LC–MS detected microcystins less frequently than ELISA and LC–MS/MS, which can be attributed to (1) a higher MDL for LC–MS (table 5) and (2) the ability of ELISA to detect a larger number of congeners. The MDL of LC–MS was about 40 times greater than that of LC–MS/MS in this study, which means that the LC–MS/MS can detect much lower concentrations. The better LC–MS/MS sensitivity in comparison to LC–MS is due to the tandem capability of LC–MS/MS, which gives a lower background noise, and other improvements in instrumentation that have occurred over the past 20 years (when the LC–MS used for this study was manufactured). Thus, the LC–MS/MS could detect compounds at low concentrations that might be missed by the LC–MS method. However, there was a strong linear correlation (Pearson’s correlation coefficient; r=0.95) between the two mass spectrometry methods for all matched sample results originating from the same study lake, depth, and deployment period (fig. 3). Mean microcystins concentration measured by LC–MS/MS was about three and a half times higher than the mean concentration measured by LC–MS (table 7). The strong linear correlation was not substantially affected by preservation status (table 7; fig. 3). Use of the ELISA preservative affected the absolute microcystins concentration; in a comparison of 10 paired preserved and unpreserved samples, overall, microcystins concentrations measured by LC–MS and LC–MS/MS in preserved samples were higher than in unpreserved samples, by an average of about 35 and 47 percent, respectively (fig. 4).
Table 6.
Summary of microcystins detected in all solid phase adsorption toxin tracking extract samples by three analytical methods.[Data from Stouder and others (2024); National Water Information System (USGS, 2016). µg/L. microgram per liter; ELISA, enzyme-linked immunosorbent assay; LC–MS, liquid chromatography with mass spectrometry; LC–MS/MS, liquid chromatography with tandem mass spectrometry]
Table 7.
Summary of microcystins detected in paired solid phase adsorption toxin tracking extract samples by two mass spectrometry methods.[Data from Stouder and others (2024); National Water Information System (USGS, 2016). µg/L, microgram per liter; LC–MS, liquid chromatography with mass spectrometry; LC–MS/MS, liquid chromatography with tandem mass spectrometry]
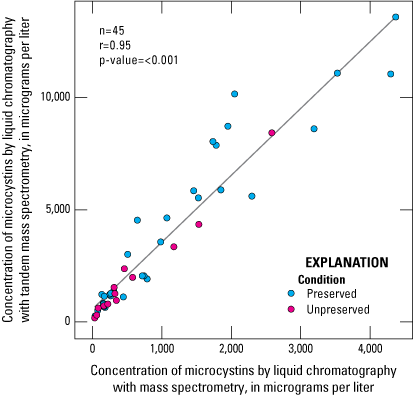
Scatterplot showing linear relation between microcystins concentration determined by liquid chromatography with mass spectrometry (LC–MS) and liquid chromatography with tandem mass spectrometry (LC–MS/MS). Data from Stouder and others (2024); National Water Information System (USGS, 2016).
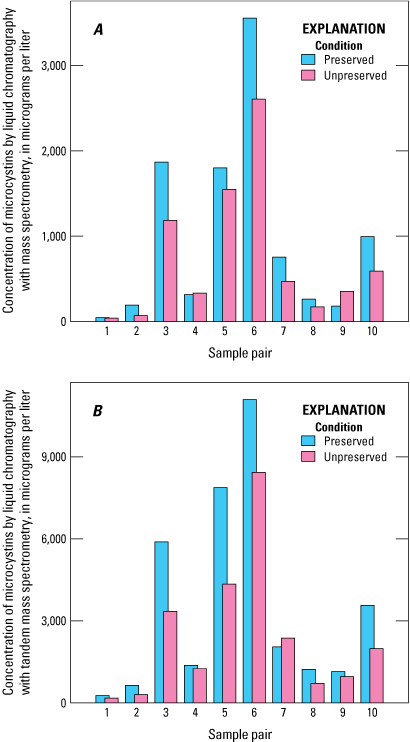
Bar graphs comparing microcystins concentration of 10 paired preserved and unpreserved samples determined using mass spectrometry methods: A, liquid chromatography with mass spectrometry (LC–MS); B, liquid chromatography with tandem mass spectrometry (LC–MS/MS). Data from Stouder and others (2024); National Water Information System (USGS, 2016).
ELISA results have a strong linear correlation with results from both LC–MS and LC–MS/MS (LC–MS r=0.90; LC–MS/MS r=0.95; fig. 5). Concentrations of microcystins measured by ELISA were consistently higher than those measured by LC–MS or LC–MS/MS (fig. 5; table 8), which is similar to findings in other studies that compared concentrations measured by ELISA to LC–MS or LC–MS/MS in discrete water samples (Satchwell and Boyer, 2002; Graham and others, 2010; and Birbeck and others, 2019). The higher concentrations measured by ELISA are likely due to its ability to detect a broader range of congeners than the two mass spectrometry methods, as well as cross-reactivity with nontarget analytes.
Table 8.
Summary of microcystins concentration measured in the same solid phase adsorption toxin tracking extract samples by three analytical methods.[Data from Stouder and others (2024); National Water Information System (USGS, 2016). µg/L, microgram per liter; ELISA, enzyme-linked immunosorbent assay; LC–MS, liquid chromatography with mass spectrometry; LC–MS/MS, liquid chromatography with tandem mass spectrometry]
The mean microcystins concentration measured by ELISA was about eight times higher than the mean concentration measured by LC–MS and about two times as high as the mean concentration measured by LC–MS/MS (table 8). Although the strong linear correlations between these analysis methods were not substantially affected by preservation status (fig. 5), the differences in these ratios were smaller in preserved mass spectrometry samples and larger in unpreserved mass spectrometry samples because of higher microcystins concentrations observed in the preserved samples than in the unpreserved samples (fig. 4). These results are based on a small sample set (four unpreserved samples), so it is still uncertain whether the correlation of ELISA to mass spectrometry methods is affected by the preservation status of the samples analyzed by mass spectrometry (fig. 5).
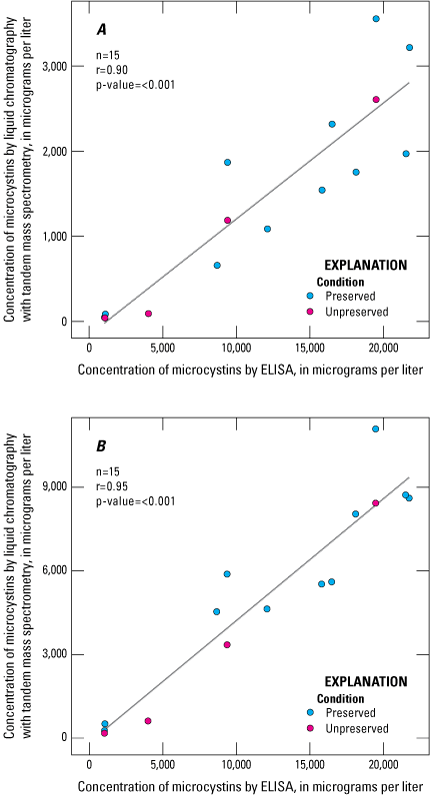
Scatterplots showing linear relation between microcystins concentrations determined using enzyme-linked immunosorbent assay (ELISA) and the microcystins concentrations determined using two mass spectrometry methods: A, liquid chromatography with mass spectrometry (LC–MS); B, liquid chromatography with tandem mass spectrometry (LC–MS/MS). Data from Stouder and others (2024); National Water Information System (USGS, 2016).
Three congeners (LR, LA, YR) were detected by LC–MS, whereas seven congeners (LR, LA, dLR, HilR, LY, YR, LF) were detected by LC–MS/MS (table 9). This difference is attributed to the lower MDL of LC–MS/MS, and thus its ability to detect congeners at much lower concentrations. The addition of preservative—which is required for analysis by ELISA of anatoxins and saxitoxins—did not result in substantial differences in the detection and quantification of relative percentage of microcystin congeners by mass spectrometry methods (table 10). Relative percentage is the unit provided by the mass spectrometry methods for microcystins. Microcystin congeners LR and LA were the most common congeners detected in the pairs of preserved and unpreserved samples; LR was detected in all samples regardless of method or preservation status (table 10).
Table 9.
Summary of relative contribution of microcystin congeners to total microcystin concentration in solid phase adsorption toxin tracking (SPATT) extract samples analyzed by two mass spectrometry methods.[Data from Stouder and others (2024); National Water Information System (USGS, 2016). Refer to table 2 for microcystin congeners]
Table 10.
Summary of relative contribution of microcystin congeners to total microcystin concentration in pairs of preserved and unpreserved solid phase adsorption toxin tracking (SPATT) extract samples by two mass spectrometry methods.[Data from Stouder and others (2024); National Water Information System (USGS, 2016). Refer to table 2 for microcystin congeners]
Results of Anatoxins Analyses
Anatoxins were detected in all the SPATT extract samples analyzed by ELISA but only in about 30 percent of samples analyzed by LC–MS/MS (table 5). This discrepancy between analysis methods is attributed primarily to dissolved organic matter interference—nonspecific adsorption of nontarget analytes, which can result in inaccurate cyanotoxin quantification—as was discussed previously for cylindrospermopsins and saxitoxins. Of the 95 SPATT samples analyzed, 55 measurements by ELISA and 26 by LC–MS/MS were considered satisfactory for consideration in these results (table 11).
Table 11.
Summary of total anatoxin concentrations of solid phase adsorption toxin tracking extract samples by two analytical methods.[Data from Stouder and others (2024); National Water Information System (USGS, 2016). µg/L, microgram per liter; ELISA, enzyme-linked immunosorbent assay; LC–MS/MS, liquid chromatography with tandem mass spectrometry]
Anatoxin concentrations by ELISA had a strong linear correlation to anatoxin concentrations by LC–MS/MS analysis (r=0.90; fig. 6). Anatoxins concentrations measured by ELISA are generally higher than those measured by LC–MS/MS, by an average of about 55 percent (table 12; fig. 6). Preservation status affected the absolute anatoxins concentration; in a comparison of eight paired preserved and unpreserved samples, overall, anatoxins concentrations measured by LC–MS/MS in the preserved samples were higher than in unpreserved samples by an average of about 31 percent (fig. 7). Although figure 6 shows there is a strong linear correlation between ELISA and LC–MS/MS results for anatoxins, these results are heavily influenced by two pairs of preserved and unpreserved samples. When those two pairs are not considered, the correlation between ELISA and LC–MS/MS anatoxin concentration is very weak (r=0.03). Furthermore, the sample set included just four unpreserved samples that were available for analysis, complicating efforts to determine whether the correlation is influenced by preservation status in the samples analyzed by mass spectrometry (fig. 6).
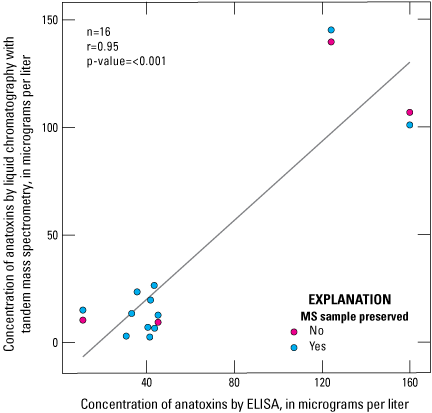
Scatterplot showing linear relation between anatoxin concentrations as determined by using enzyme-linked immunosorbent assay (ELISA) and liquid chromatography with tandem mass spectrometry (LC–MS/MS) methods. Data from Stouder and others (2024); National Water Information System (USGS, 2016).
Table 12.
Summary of total anatoxin concentrations measured in the same solid phase adsorption toxin tracking (SPATT) extract samples by two analytical methods.[Data from Stouder and others (2024); National Water Information System (USGS, 2016). µg/L, microgram per liter; ELISA, enzyme-linked immunosorbent assay; LC–MS/MS, liquid chromatography with tandem mass spectrometry]
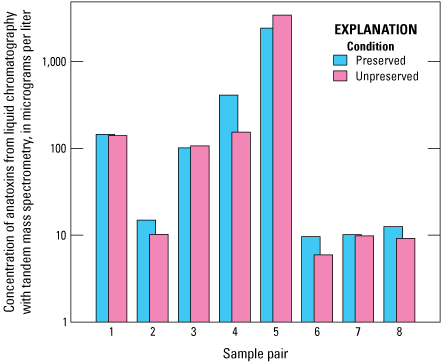
Bar graph comparing anatoxin concentrations of eight paired preserved and unpreserved samples as determined by using liquid chromatography with tandem mass spectrometry (LC–MS/MS). Data from Stouder and others (2024); National Water Information System (USGS, 2016).
Three of the four measured congeners (homoanatoxin-a, dihydrohomoanatoxin-a, and dihydroanatoxin-a) were detected; however, anatoxin-a was absent in all samples (table 13). This anomaly may contribute to the discrepancy observed between sample results of ELISA and LC–MS/MS; the anatoxin ELISA antibody is specific to anatoxin-a and has varying levels of cross- reactivity to these other congeners (Gold Standard Diagnostics, 2024a). The addition of preservative that is required for analysis by ELISA of anatoxins and saxitoxins did not result in substantial differences in the detection and quantification of concentrations of anatoxin congeners by LC–MS/MS, except for an increase in the maximum, mean, and median homoanatoxin-a concentrations (table 14). Overall, dihydroanatoxin-a is the most common congener detected in all the LC–MS/MS samples, whereas homoanatoxin-a is the congener with the highest concentrations (tables 13 and 14).
Table 13.
Summary of concentrations of anatoxin congeners in solid phase adsorption toxin tracking (SPATT) extract samples analyzed by liquid chromatography with tandem mass spectrometry.[Data from Stouder and others (2024); National Water Information System (USGS, 2016). µg/L, microgram per liter; —, not applicable]
Table 14.
Summary of concentrations of anatoxin congeners in pairs of preserved and unpreserved solid phase adsorption toxin tracking (SPATT) extract samples analyzed by mass spectrometry.[Data from Stouder and others (2024); National Water Information System (USGS, 2016). µg/L, microgram per liter; —, not applicable]
Comparative Analysis of SPATT and Discrete Sample Results
Discrete samples generally represent the concentration of cyanotoxins at a specific time and depth. They provide instantaneous measurements of either total (intracellular and dissolved), particulate (intracellular), or dissolved cyanotoxins representing a single point in time in a waterbody. In contrast, SPATT samplers integrate concentrations over time, providing measurements of accumulated cyanotoxins, albeit only in their dissolved form.
Because SPATT samples are a cumulative measure, daily mean cyanotoxin concentrations (calculated by dividing SPATT sample concentrations by the number of days deployed) were used to compare to discrete samples that were collected during each SPATT sample recovery. Comparison of SPATT extract samples and discrete samples collected over the duration of this study demonstrates that SPATT samplers provided a quantifiable amount of both microcystins and anatoxins more often than discrete samples in the study lakes, as indicated by the higher frequency of measurements above the minimum reporting level (figs. 8–11). For microcystins, about 40 percent of SPATT extract samples analyzed by ELISA, and about 70 percent analyzed by LC–MS, showed cyanotoxin detections that were not observed in the corresponding discrete samples. Similarly, about 80 percent of SPATT extract samples had positive detections for anatoxins by ELISA, whereas no detections above the minimum reporting level were observed in the corresponding discrete samples. The LC–MS/MS results for anatoxins show that about 30 percent of SPATT extract samples indicated cyanotoxin detections that were not observed in the corresponding discrete samples. These results show that SPATT sampling is useful for capturing cyanotoxins that may be missed by the point-in-time approach of discrete samples, particularly if they are unevenly distributed with depth and time.
Overall, the comparative analysis shows that whereas discrete sampling is useful for understanding the immediate state of a waterbody, SPATT samplers can provide a more comprehensive overview of cyanotoxin presence over time. Thus, SPATT samplers may be a more reliable indicator of long-term cyanotoxin exposure and the potential ecological and health risks. For ongoing water quality monitoring, especially in environments where cyanotoxin concentrations are expected to vary or have blooms that are ephemeral in nature, incorporating SPATT sampling could enhance the detection and understanding of harmful algal blooms and their associated cyanotoxins.
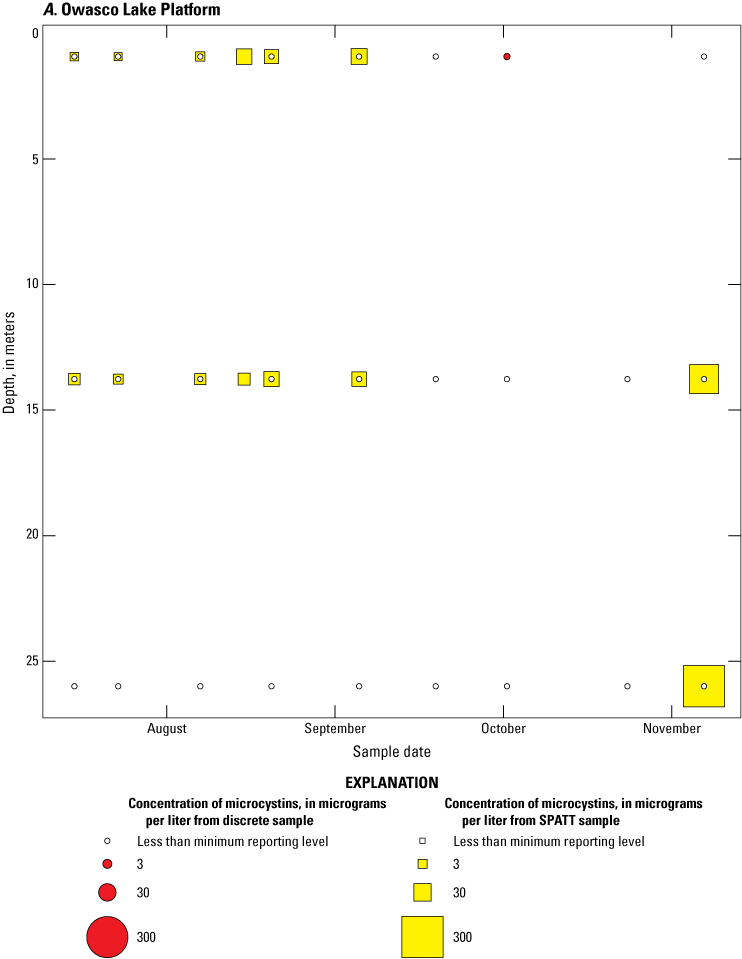
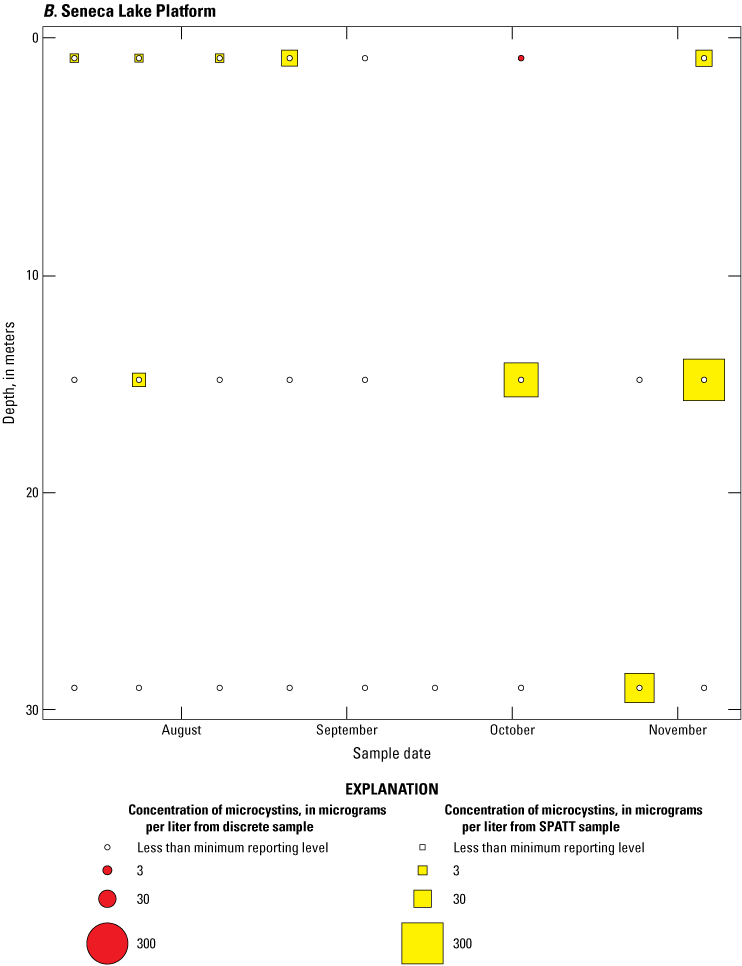
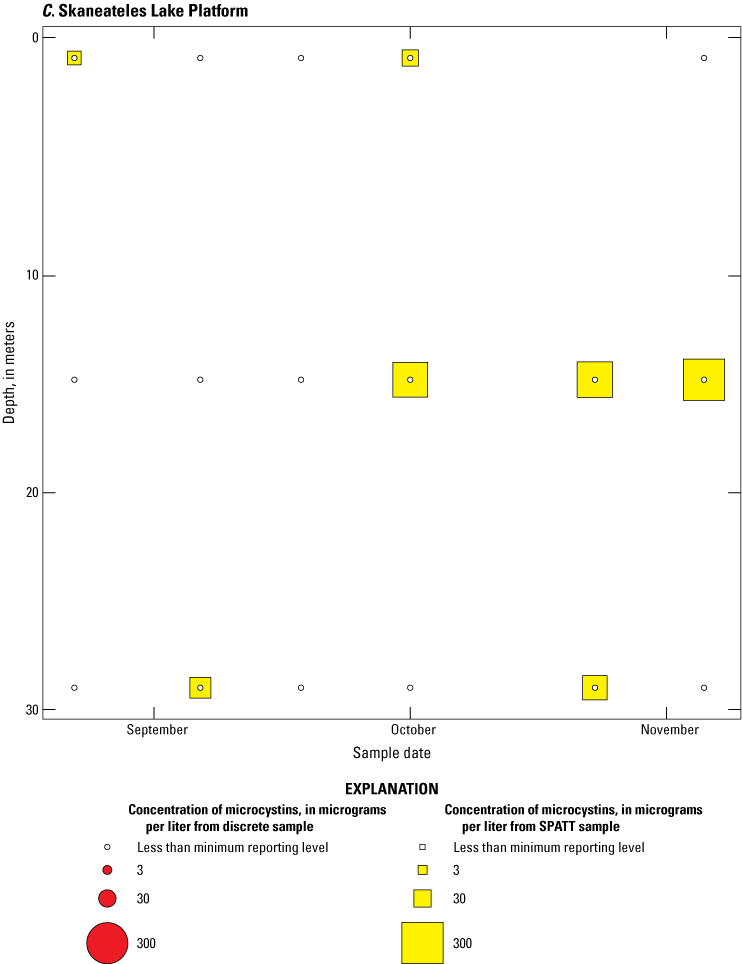
Bubble plots of temporal and depth variation in microcystin concentrations analyzed by enzyme-linked immunosorbent assay (ELISA) in discrete and solid phase adsorption toxin tracking (SPATT) samples for A, Owasco Lake; B, Seneca Lake; and C, Skaneateles Lake. Discrete microcystin concentration are given as instantaneous values; SPATT microcystin concentrations are given as daily mean values. Data from Stouder and others (2024); National Water Information System (USGS, 2016).
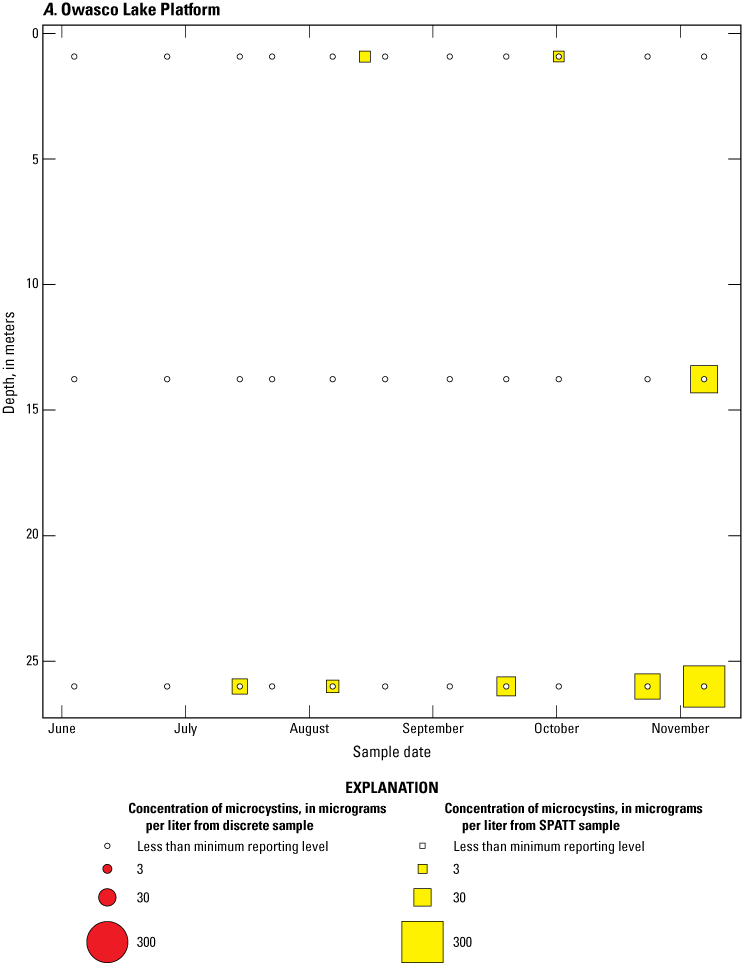
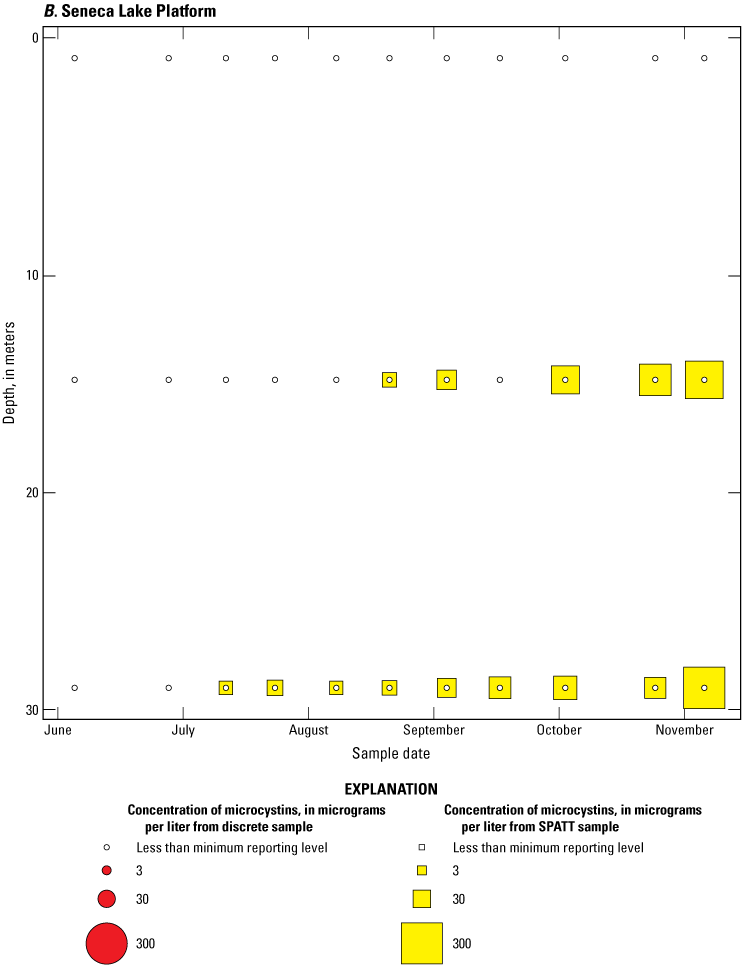
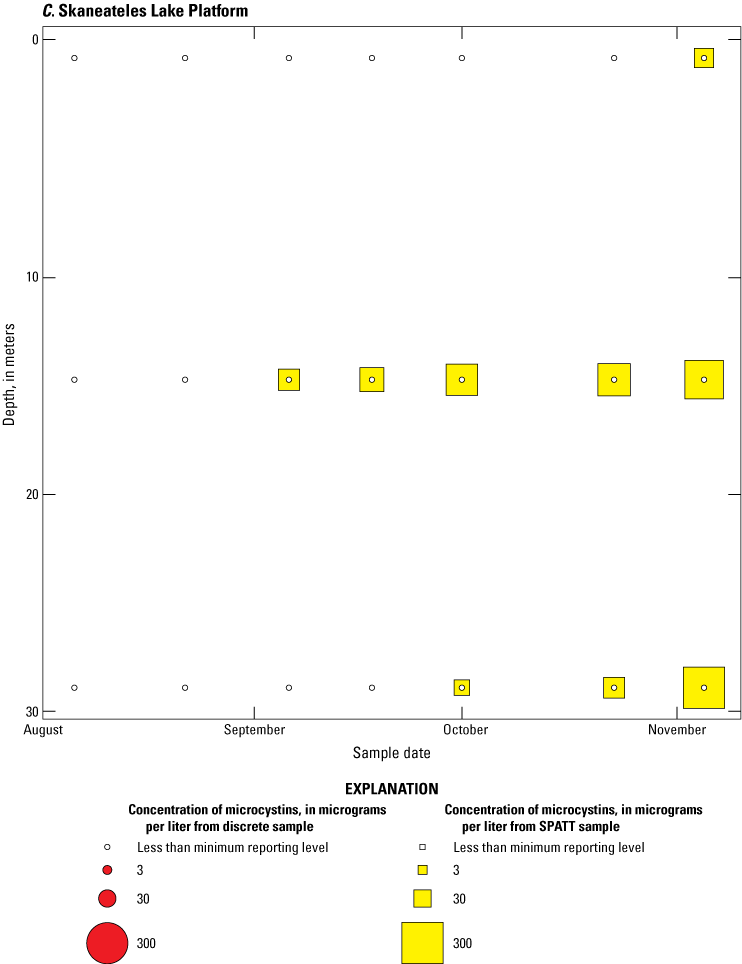
Bubble plots of temporal and depth variation in microcystin concentrations analyzed by liquid chromatography with mass spectrometry in discrete and solid phase adsorption toxin tracking (SPATT) samples for A, Owasco Lake; B, Seneca Lake; and C, Skaneateles Lake. Discrete microcystin concentration are given as instantaneous values; SPATT microcystin concentrations are given as daily mean values. Data from Stouder and others (2024); National Water Information System (USGS, 2016).
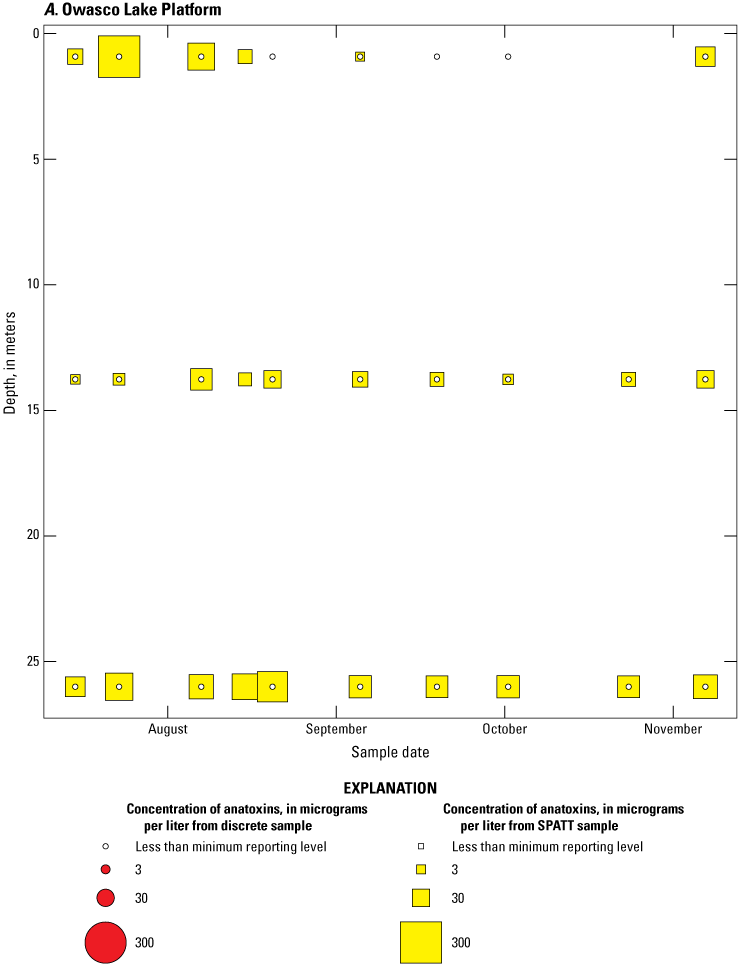
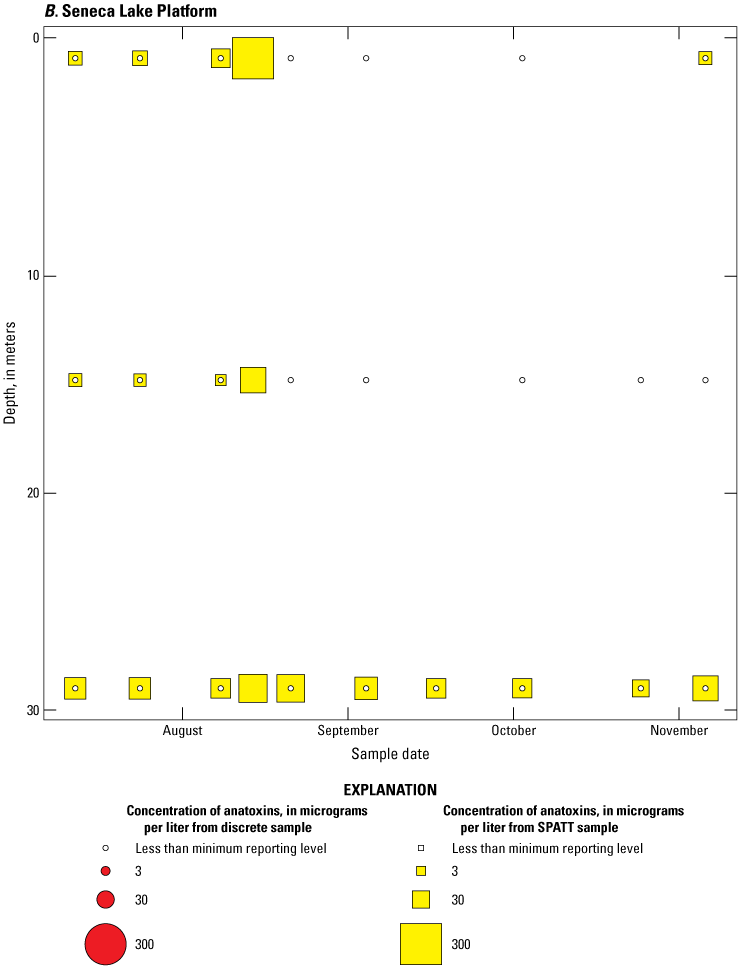

Bubble plots of temporal and depth variation in anatoxin concentrations analyzed by enzyme-linked immunosorbent assay in discrete and solid phase adsorption toxin tracking (SPATT) samples for A, Owasco Lake; B, Seneca Lake; C, Skaneateles Lake. Discrete anatoxin concentration are given as instantaneous values; SPATT anatoxin concentrations are given daily mean values. Data from Stouder and others (2024); National Water Information System (USGS, 2016).
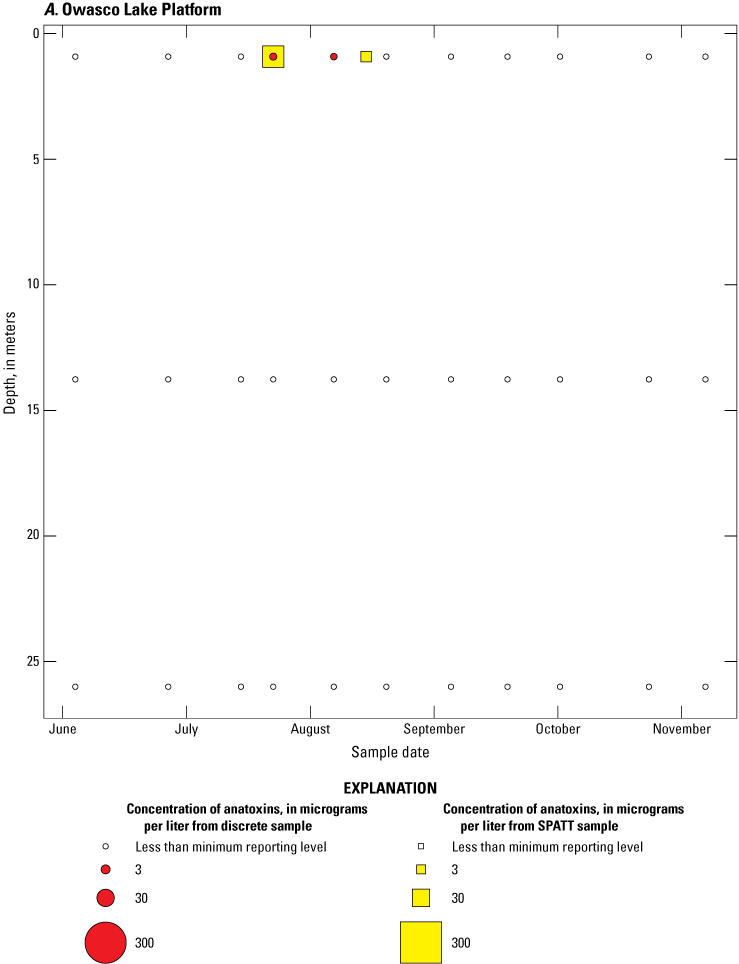
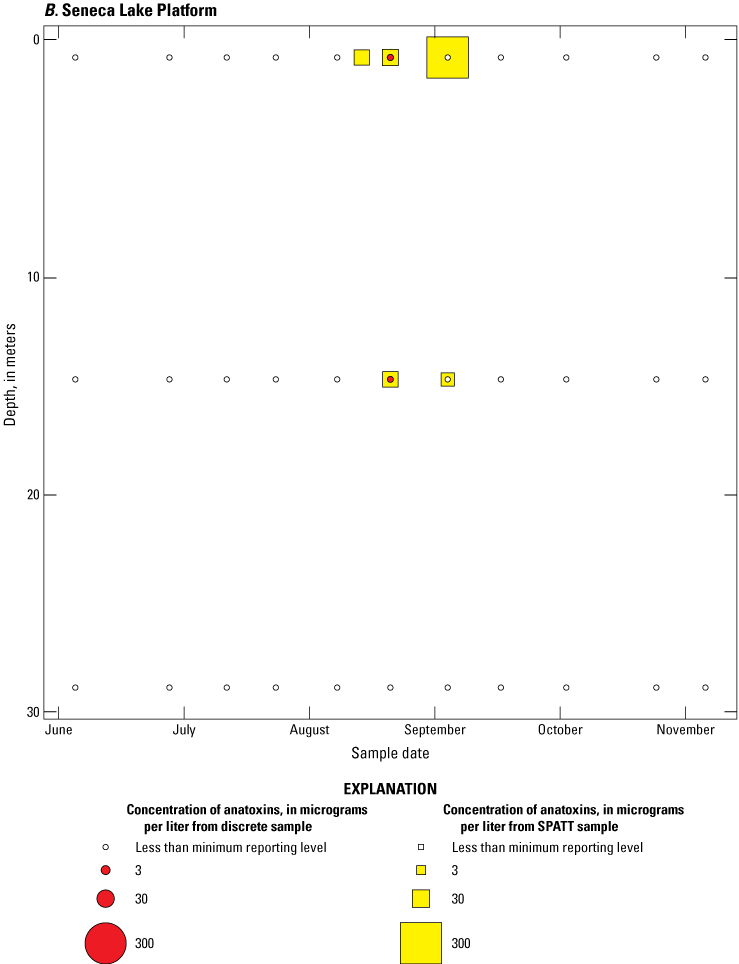

Bubble plots of temporal and depth variation in anatoxin concentrations analyzed by liquid chromatography with tandem mass spectrometry in discrete and solid phase adsorption toxin tracking (SPATT) samples for A, Owasco Lake; B, Seneca Lake; and C, Skaneateles Lake. Discrete anatoxin concentration are given as instantaneous values; SPATT anatoxin concentrations are given as daily mean values. Data from Stouder and others (2024); National Water Information System (USGS, 2016).
Discussion of SPATT Efficacy and Cyanotoxin Detection
The findings from this study offer updated insights into the application of various methods for the analysis of SPATT extract samples for monitoring cyanotoxins in freshwater ecosystems. Specifically, the study examines the effects of preservatives, which are necessary for some cyanotoxin analyses by ELISA, on the results of mass spectrometry analyses. This study highlights both the potential and the challenges associated with using these techniques for accurate cyanotoxin detection.
The extensive detection of microcystins by all three methods—ELISA, LC–MS, and LC–MS/MS—confirms the presence of this cyanotoxin in the study lakes during the study period. Microcystins were detected less frequently with LC–MS than the other two methods, highlighting the critical role of method sensitivity in cyanotoxin detection (Boyer, 2020). Whereas LC–MS/MS methods have a lower detection limit, they require calibration using specific cyanotoxin standards to optimize the fragmentation voltages that are critical for congener detection; hence the congeners measured by LC–MS/MS are limited to those congeners for which standards are available. In contrast, LC–MS methods can use a generic ionization voltage (a standard energy level applied to ionize compounds) that, when coupled with photodiode array detection (a detector that measures how compounds absorb light), allows for detection of many more congeners than LC–MS/MS methods. In this case, the primary microcystin congeners detected were LR, LA, and YR, which are commonly included in both methods. But these three microcystin congeners may not always represent the highest cyanotoxin concentrations in algal blooms. For example, Planktothrix blooms often produce dimethyl derivatives, but dimethyl derivatives are not included in common LC–MS/MS methods. Similarly, benthic microcystin producers may have a different congener profile, leading to different results (Briand and others, 2005). These limitations highlight the complementary nature of LC–MS and LC–MS/MS approaches for comprehensive analysis of microcystins.
When microcystins were detected in a sample by ELISA and one or both mass spectrometry methods, the concentrations measured by ELISA were always higher than those obtained by mass spectrometry. This is partially attributable to the fact that the mass spectrometry methods can only identify the limited number of congeners used in the analytical protocol, whereas ELISA can detect any congener that has reactivity with the ADDA-specific antibody used (Gold Standard Diagnostics, 2024c). However, it is also likely that the high concentrations of dissolved organic matter present in the extract samples caused some form of positive bias in the analytical concentration and (or) congener-relative percentage results, as was discussed previously for cylindrospermopsins and saxitoxins.
The correlation between ELISA and LC–MS/MS concentrations of anatoxins, although strong, was based on a limited set of preserved and unpreserved sample pairs, indicating a need for larger datasets to verify these results. Preservation is required for anatoxins or saxitoxins analyses by ELISA, and preservation status substantially influenced microcystins concentrations measured by both LC–MS and LC–MS/MS, with preserved samples resulting in higher concentrations than unpreserved samples. Whereas extracts preserved for anatoxins or saxitoxins analyses by ELISA can be analyzed for microcystins by mass spectroscopy, the presence of preservatives may alter the detected concentrations. Thus, some consideration should be given to preservation status if confirmatory analysis by mass spectrometry is planned. Because the preservative is proprietary, it is unknown why it may affect concentrations measured by mass spectrometry methods. Three findings of this study suggest limitations in the current ELISA methods: the positive bias found in the microcystins ELISA results; the detection of anatoxins in all samples by ELISA, only some of which were confirmed by LC–MS/MS; and the lack of cylindrospermopsins and saxitoxins detections confirmed by LC–MS/MS. ELISA outcomes are also known to be affected by potential interference from dissolved organic matter (Nunes and others, 1998; Huang and Sedlak, 2001; Hanselman and others, 2004; Silva and others, 2014).
For ELISA to be a feasible technique for SPATT extract analysis, further research is needed to determine the nature of, and possible solutions for, matrix interference (Hanselman and others, 2004). Whereas the simplest solution to this problem is to dilute or reconstitute the sample in a greater volume of solvent to reduce the interference of solutes other than the analyte (Nunes and others, 1998), this has the unwanted side effect of reducing analysis sensitivity to the analyte. Previous studies with immunoassay techniques for environmental sampling have used various clean-up techniques such as solid phase extraction and liquid chromatography (Huang and Sedlak, 2001), liquid-liquid extraction (Hanselman and others, 2004), or the addition of bovine serum albumin to the assay, all of which have been shown to greatly reduce the interference from dissolved organic matter as well as dissolved salts (Silva and others, 2014). If the matrix interference issues can be solved, using ELISA on SPATT extracts would offer the benefits of simplicity, sensitivity, the ability to process large numbers of samples quickly, as well as lower equipment and training costs compared to mass spectrometry (Nunes and others, 1998; Huang and Sedlak, 2001; Silva and others, 2014), making ELISA a promising monitoring option (Hattenrath-Lehmann and others, 2018).
If ELISA is to be used as the sole method for quantifying cylindrospermopsins in natural waters, the small number of known congeners (three) limits potential cross-reactivity and simplifies method development. This limited number of cylindrospermopsin congeners also simplifies LC–MS/MS methods, which can be developed to detect these congeners with high sensitivity. This is not the case for saxitoxins where the number of congeners potentially present in freshwater systems is large (greater than 57; Wiese and others, 2010), which complicates both mass spectrometry detection methods and causes cross-reactivity issues with ELISA.
The analysis across the three lakes using both SPATT and discrete samples provides a robust dataset. Notably, cyanotoxin classes microcystins and anatoxins were detected above the minimum reporting level in SPATT samples more frequently than concurrently collected discrete samples. SPATT sampling may indicate the prevalence of transient cyanobacteria populations, the movement of cyanotoxins around the lake, or low concentration that accumulate over time and are not detectable with discrete sampling alone. The microcystins and anatoxins concentrations varied with time and depth, demonstrating the necessity of both time-integrated and discrete sampling methods for a comprehensive assessment of cyanotoxin occurrence. This comparison between sampling and analysis methods has implications for environmental monitoring programs, which aim to safeguard public health and the integrity of aquatic ecosystems, and drinking water providers that take water in at depth (Prestigiacomo and others 2023).
Summary
Cyanobacterial harmful algal blooms (cyanoHABs) pose significant threats to aquatic ecosystems, through reductions in dissolved oxygen and light availability, and to human health, by affecting drinking water supplies and recreational resources. In response to increasing occurrences of cyanoHABs, the U.S. Geological Survey, in collaboration with the New York State Department of Environmental Conservation, conducted a cyanoHAB advanced monitoring pilot study in the Finger Lakes region of central New York from 2018 to 2020. The study aimed to evaluate traditional and innovative monitoring approaches to improve understanding and management of cyanoHABs.
In 2019, the U.S. Geological Survey deployed solid phase adsorption toxin tracking (SPATT) samplers in Seneca Lake, Owasco Lake, and Skaneateles Lake to monitor four classes of cyanotoxins: microcystins, cylindrospermopsins, anatoxins, and saxitoxins. SPATT samplers are passive environmental sampling devices that adsorb dissolved cyanotoxins, offering time-integrated data on cyanotoxin presence, including low concentrations that may go undetected with traditional discrete sampling. However, unlike discrete samples, SPATT samplers only adsorb dissolved toxins and are influenced by environmental factors, such as variations in flow velocity and cyanotoxin concentration over the deployment period. Discrete samples can provide measures of both dissolved and intracellular cyanotoxins at specific times and depths, offering instantaneous but isolated views of water conditions.
SPATT samplers were deployed at multiple depths and periodically retrieved for analysis by using enzyme-linked immunosorbent assay (ELISA), liquid chromatography-mass spectrometry (LC–MS), and tandem mass spectrometry (LC–MS/MS). Discrete water samples were collected concurrently with SPATT retrievals and analyzed with the same three methods to compare and interpret the SPATT results. ELISA results often showed higher cyanotoxin concentrations compared to LC–MS and LC–MS/MS results, likely due to matrix interference from dissolved organic matter and the ability of ELISA to detect a broader range of congeners not identified by the two mass spectrometry methods. For concentrations of microcystins, strong correlations were observed between ELISA and LC–MS/MS results. ELISA and LC–MS/MS detected microcystins in 100 percent of the samples, whereas LC–MS detected microcystins in approximately 66 percent of the samples—70 percent of preserved samples and 63 percent of unpreserved samples. Preservation substantially increased the measured microcystins concentrations, suggesting that the preservatives used for the ELISA may affect mass spectrometry analyses.
ELISA detected anatoxins in 100 percent of samples, whereas LC–MS/MS detected anatoxins in only about 32 percent—35 percent of preserved samples and 30 percent of unpreserved samples. This discrepancy suggests that dissolved organic matter interference likely affected the ELISA results for concentrations of anatoxins, causing higher detection rates compared to the more specific LC–MS/MS method. Preservation status did not substantially influence anatoxins concentrations measured by LC–MS/MS, demonstrating that additional research would be needed to better understand the factors affecting ELISA accuracy for anatoxins detection. Whereas both microcystins and anatoxins were found in higher concentrations in ELISA results, the effect of preservation status was more substantial for microcystins than anatoxins in LC–MS/MS analyses.
This study highlights limitations in current ELISA methods for detecting cylindrospermopsins and saxitoxins, with potential false positives attributed to dissolved organic matter interference. Dissolved organic matter was evident by yellow-brown coloration observed in SPATT extract samples. Additionally, no confirmatory detections of cylindrospermopsins were made by LC–MS/MS, casting doubt on the accuracy of the ELISA results. No mass spectrometry analyses were performed for saxitoxins because of inconsistencies in published methods. Given these strong indicators of interference and the lack of confirmatory data, the ELISA detections of cylindrospermopsins and saxitoxins were deemed unreliable and were not discussed in the results.
The comparative analysis indicated that cyanotoxins were detected more frequently in SPATT samples than in the discrete samples. Microcystins and anatoxins concentrations from SPATT extracts exceeded the upper limit of ELISA calibration curves in 100 and 94 percent of samples, respectively. In contrast, discrete samples collected concurrently with SPATT retrievals showed much lower detection frequencies. For microcystins, 40 percent of SPATT samples analyzed by ELISA and 70 percent analyzed by LC–MS showed detections not observed in discrete samples. Similarly, 80 percent of SPATT samples had positive detections for anatoxins by ELISA, with no detections in discrete samples, while 30 percent of SPATT samples showed anatoxin detections by LC–MS/MS not observed in discrete samples. This is likely due to the sensitivity of SPATT samplers and their ability to concentrate low levels of cyanotoxins over time. Whereas discrete sampling is used for capturing instantaneous conditions, SPATT samplers may provide a more reliable indicator of long-term cyanotoxin exposure and the corresponding potential ecological and health risks. The findings of this study support the incorporation of SPATT sampling into ongoing water quality monitoring programs, especially in environments with variable cyanotoxin concentrations or ephemeral blooms. This study provides insights into the advantages and limitations of SPATT samplers for cyanotoxin monitoring and identifies areas in which further research could improve the reliability of ELISA and other analytical methods for detecting cyanotoxins in freshwater ecosystems.
References Cited
Barnard, M.A., Chaffin, J.D., Plaas, H.E., Boyer, G.L., Wei, B., Wilhelm, S.W., Rossignol, K.L., Braddy, J.S., Bullerjahn, G.S., Bridgeman, T.B., Davis, T.W., Wei, J., Bu, M., and Paerl, H.W., 2021, Roles of nutrient limitation on western Lake Erie cyanoHAB toxin production: Toxins, v. 13, no. 1, article 47, 21 p., accessed July 19, 2023, at https://doi.org/10.3390/toxins13010047.
Birbeck, J.A., Westrick, J.A., O’Neill, G.M., Spies, B., and Szlag, D.C., 2019, Comparative analysis of microcystin prevalence in Michigan lakes by online concentration LC/MS/MS and ELISA: Toxins, v. 11, no. 1, article 13, 16 p., accessed July 21, 2023, at https://doi.org/10.3390/toxins11010013.
Boyer, G.L., 2007, The occurrence of cyanobacterial toxins in New York lakes—Lessons from the MERHAB-Lower Great Lakes program: Lake and Reservoir Management, v. 23, no. 2, p. 153–160, accessed July 25, 2023, at https://doi.org/10.1080/07438140709353918.
Boyer, G.L., 2020, LCMS–SOP determination of microcystins in water samples by high performance liquid chromatography (HPLC) with single quadrupole mass spectrometry (MS): protocols.io article, 33 p., accessed July 19, 2023, at https://dx.doi.org/10.17504/protocols.io.bck2iuye.
Briand, J.F., Jacquet, S., Flinois, C., Avois-Jacquet, C., Maisonnette, C., Leberre, B., and Humbert, J.F., 2005, Variations in the microcystin production of Planktothrix rubescens (Cyanobacteria) assessed from a four-year survey of Lac du Bourget (France) and from laboratory experiments: Microbial Ecology, v. 50, no. 3, p. 418–428, accessed May 31, 2024, at https://doi.org/10.1007/s00248-005-0186-z.
Callinan, C.W., 2001, Water quality study of the Finger Lakes: New York State Department of Environmental Conservation report, 152 p., accessed July 19, 2023, at https://nysl.ptfs.com/data/Library1/Library1/pdf/50277116.pdf.
Carpenter, K.D., and Wise, D.R., 2023, Cyanotoxin concentrations in extracts from cyanobacteria colonies, plankton net tows, and solid phase adsorption toxin tracking (SPATT) samplers in western rivers, lakes, and reservoirs, including drinking water sources in the Oregon Cascades—2016–2020: U.S. Geological Survey data release, accessed August 29, 2024, at https://doi.org/10.5066/P96VPGH7.
Conklin, K.Y., Stancheva, R., Otten, T.G., Fadness, R., Boyer, G.L., Read, B., Zhang, X., and Sheath, R.G., 2020, Molecular and morphological characterization of a novel dihydroanatoxin-a producing Microcoleus species (cyanobacteria) from the Russian River, California, USA: Harmful Algae, v. 93, article e101767, accessed February 4, 2025, at https://doi.org/10.1016/j.hal.2020.101767.
Dodds, W.K., Bouska, W.W., Eitzmann, J.L., Pilger, T.J., Pitts, K.L., Riley, A.J., Schloesser, J.T., and Thornbrug, D.J., 2009, Eutrophication of U.S. freshwaters—Analysis of potential economic damages: Environmental Science & Technology, v. 43, no. 1, p. 12–19, accessed July 25, 2023, at https://doi.org/10.1021/es801217q.
Favot, E.J., Holeton, C., DeSellas, A.M., and Paterson, A.M., 2023, Cyanobacterial blooms in Ontario, Canada—Continued increase in reports through the 21st century: Lake and Reservoir Management, v. 39, no. 1, p. 1–20, accessed May 29, 2024, at https://doi.org/10.1080/10402381.2022.2157781.
Foss, A.J., Phlips, E.J., Yilmaz, M., Chapman, A., 2012, Characterization of paralytic shellfish toxins from Lyngbya wollei dominated mats collected from two Florida springs: Harmful Algae, v. 16, p. 98–107, accessed May 31, 2024, at https://doi.org/10.1016/j.hal.2012.02.004.
Gaget, V., Lau, M., Sendall, B., Froscio, S., and Humpage, A.R., 2017, Cyanotoxins—Which detection technique for an optimum risk assessment?: Water Research, v. 118, p. 227–238, accessed March 22, 2023, at https://doi.org/10.1016/j.watres.2017.04.025.
Gold Standard Diagnostics, 2024a, ABRAXIS® anatoxin-a ELISA microtiter plate [user guide] (ver. 06): Warminster, Pa., Gold Standard Diagnostics, 4 p., accessed May 9, 2024, at https://www.goldstandarddiagnostics.us/media/16937/ug-21-057-rev-06-abraxis-anatoxin-a-elisa_520060.pdf.
Gold Standard Diagnostics, 2024b, ABRAXIS® cylindrospermopsin ELISA microtiter plate [user guide] (ver. 04): Warminster, Pa., Gold Standard Diagnostics, 4 p., accessed May 9, 2024, at https://www.goldstandarddiagnostics.us/media/16949/ug-21-059-rev-04-abraxis-cylindrospermopsin-elisa_522011.pdf.
Gold Standard Diagnostics, 2024c, ABRAXIS® microcystin-ADDA ELISA microtiter plate [user guide] (ver. 03): Warminster, Pa., Gold Standard Diagnostics, 4 p., accessed May 9, 2024, at https://www.goldstandarddiagnostics.us/media/16938/ug-21-052-rev-03-abraxis-microcystins-adda-elisa_520011.pdf.
Gold Standard Diagnostics, 2024d, ABRAXIS® saxitoxin (PSP) ELISA microtiter plate [user guide] (ver. 05): Warminster, Pa., Gold Standard Diagnostics, 4 p., accessed May 9, 2024, at https://www.goldstandarddiagnostics.us/media/16933/ug-21-081-rev-05-abraxis-saxitoxin-elisa_52255b.pdf.
Gorney, R.M., June, S.G., Stainbrook, K.M., and Smith, A.J., 2023, Detections of cyanobacteria harmful algal blooms (cyanoHABs) in New York State, United States (2012–2020): Lake and Reservoir Management, v. 39, no. 1, p. 21–36, accessed March 6, 2024, at https://doi.org/10.1080/10402381.2022.2161436.
Graham, J.L., Loftin, K.A., Meyer, M.T., and Ziegler, A.C., 2010, Cyanotoxin mixtures and taste-and-odor compounds in cyanobacterial blooms from the Midwestern United States: Environmental Science & Technology, v. 44, no. 19, p. 7361–7368, accessed May 29, 2024, at https://doi.org/10.1021/es1008938.
Graham, J.L., Loftin, K.A., Ziegler, A.C., and Meyer, M.T., 2008, Cyanobacteria in lakes and reservoirs—Toxin and taste-and-odor sampling guidelines: U.S. Geological Survey Techniques of Water-Resources Investigations, book 9, chap. A7.5, 65 p., accessed February 28, 2023, at https://doi.org/10.3133/twri09A7.5.
Halfman, J.D., Shaw, J., Dumitriu, I., and Cleckner, L.B., 2023, Meteorological and limnological precursors to cyanobacterial blooms in Seneca and Owasco Lakes, New York, USA: Water, v. 15, no. 13, article 2363, 26 p., accessed May 29, 2024, at https://doi.org/10.3390/w15132363.
Hanselman, T.A., Graetz, D.A., and Wilkie, A.C., 2004, Comparison of three enzyme immunoassays for measuring 17β-estradiol in flushed dairy manure wastewater: Journal of Environmental Quality, v. 33, no. 5, p. 1919–1923, accessed May 29, 2024, at https://doi.org/10.2134/jeq2004.1919.
Hattenrath-Lehmann, T.K., Lusty, M.W., Wallace, R.B., Haynes, B., Wang, Z., Broadwater, M., Deeds, J.R., Morton, S.L., Hastback, W., Porter, L., Chytalo, K., and Gobler, C.J., 2018, Evaluation of rapid, early warning approaches to track shellfish toxins associated with Dinophysis and Alexandrium blooms: Marine Drugs, v. 16, no. 1, article 28, 19 p., accessed May 29, 2024, at https://doi.org/10.3390/md16010028.
Havens, K.E., 2008, Cyanobacteria blooms—Effects on aquatic ecosystems, chap. in Hudnell, H.K., ed., Cyanobacterial harmful algal blooms—State of the science and research needs: New York, Springer, p. 733–747, accessed May 29, 2024, at https://doi.org/10.1007/978-0-387-75865-7_33.
He, X., Liu, Y.-L., Conklin, A., Westrick, J., Weavers, L.K., Dionysios, D.D., Lenhart, J.J., Mouser, P.J., Szlag, D., and Walker, H.W., 2016, Toxic cyanobacteria and drinking water—Impacts, detection, and treatment: Harmful Algae, v. 54, p. 174–193, accessed April 2, 2024, at https://doi.org/10.1016/j.hal.2016.01.001.
Helsel, D.R., Hirsch, R.M., Ryberg, K.R., Archfield, S.A., and Gilroy, E.J., 2020, Statistical methods in water resources: U.S. Geological Survey Techniques and Methods book 4, chap. A3, 458 p., accessed February 22, 2023, at https://doi.org/10.3133/tm4A3. [Supersedes USGS Techniques of Water-Resources Investigations, book 4, chap. A3, version 1.1.]
Ho, C.S., Lam, C.W.K., Chan, M.H.M., Cheung, R.C.K., Law, L.K., Lit, L.C.W., Ng, K.F., Suen, M.W.M., and Tai, H.L., 2003, Electrospray ionisation mass spectrometry—Principles and clinical applications: The Clinical Biochemist Reviews, v. 24, no. 1, p. 3–12, accessed April 2, 2024, at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1853331/.
Howard, M.D.A., Kudela, R., Caron, D., Smith, J., and Hayashi, K., 2018, Standard operating procedure for solid phase adsorption toxin testing (SPATT) assemblage and extraction of HAB toxins: Santa Cruz, Calif., University of California and University of Southern California, 14 p., accessed May 8, 2024, at https://doi.org/10.25607/OBP-845.
Howard, M.D.A., Nagoda, C., Kudela, R.M., Hayashi, K., Tatters, A., Caron, D.A., Busse, L., Brown, J., Sutula, M., and Stein, E.D., 2017, Microcystin prevalence throughout lentic waterbodies in coastal southern California: Toxins, v. 9, no. 7, 21 p., accessed April 17, 2024, at https://doi.org/10.3390/toxins9070231.
Huang, C.-H., and Sedlak, D.L., 2001, Analysis of estrogenic hormones in municipal wastewater effluent and surface water using enzyme‐linked immunosorbent assay and gas chromatography/tandem mass spectrometry: Environmental Toxicology and Chemistry, v. 20, no. 1, p. 133–139, accessed May 8, 2024, at https://doi.org/10.1002/etc.5620200114.
Hudnell, H.K., ed., 2008, Cyanobacterial harmful algal blooms—State of the science and research needs: New York, Springer, 949 p., accessed May 8, 2024, at https://doi.org/10.1007/978-0-387-75865-7.
Jaramillo, M., and O’Shea, K.E., 2019, Analytical methods for assessment of cyanotoxin contamination in drinking water sources: Current Opinion in Environmental Science & Health, v. 7, p. 45–51, accessed May 8, 2024, at https://doi.org/10.1016/j.coesh.2018.10.003.
Johnston, B.D., Finkelstein, K.M., Gifford, S.R., Stouder, M.D., Nystrom, E.A., Savoy, P., Rosen, J. J., and Jennings, M. B., 2024. Evaluation of sensors for continuous monitoring of harmful algal blooms in the Finger Lakes region, New York, 2019 and 2020: U.S. Geological Survey Scientific Investigations Report 2024–5010, 54 p., accessed May 30, 2024, at https://doi.org/10.3133/sir20245010.
Kudela, R.M., 2011, Characterization and deployment of solid phase adsorption toxin tracking (SPATT) resin for monitoring of microcystins in fresh and saltwater: Harmful Algae, v. 11, p. 117–125, accessed February 15, 2023, at https://doi.org/10.1016/j.hal.2011.08.006.
Kudela, R.M., 2017, Passive sampling for freshwater and marine algal toxins: Comprehensive Analytical Chemistry, v. 78, p. 379–409, accessed February 15, 2023, at https://doi.org/10.1016/bs.coac.2017.08.006.
Lane, J.Q., Roddam, C.M., Langlois, G.W., and Kudela, R.M., 2010, Application of solid phase adsorption toxin tracking (SPATT) for field detection of the hydrophilic phycotoxins domoic acid and saxitoxin in coastal California: Limnology and Oceanography Methods, v. 8, no. 11, p. 645–660, accessed February 16, 2023, at https://doi.org/10.4319/lom.2010.8.0645.
Li, J., and Persson, K.M., 2021, Quick detection method for paralytic shellfish toxins (PSTs) monitoring in freshwater—A review: Chemosphere, v. 265, article 128591, 14 p., accessed August 29, 2024, at https://doi.org/10.1016/j.chemosphere.2020.128591.
MacKenzie, L., Beuzenberg, V., Holland, P., McNabb, P., Selwood, A., 2004, Solid phase adsorption toxin tracking (SPATT): a new monitoring tool that simulates the biotoxin contamination of filter feeding bivalves: Toxicon, v. 44, no. 8, p. 901-918, accessed January 23, 2025, at https://doi.org/10.1016/j.toxicon.2004.08.020.
MacKenzie, L.A., 2010, In situ passive solid-phase adsorption of micro-algal biotoxins as a monitoring tool: Current Opinion in Biotechnology, v. 21, no. 3, p. 326–331, accessed February 1, 2023, at https://doi.org/10.1016/j.copbio.2010.01.013.
New York State Department of Environmental Conservation [NYSDEC], 2018, Harmful algal bloom action plan Owasco Lake: [Syracuse, N.Y.], New York State Department of Environmental Conservation report, 115 p., accessed February 1, 2023, at https://www.dec.ny.gov/docs/water_pdf/owascohabplan.pdf.
New York State Department of Environmental Conservation [NYSDEC], 2019, 2018 Finger Lakes water quality report: Syracuse, N.Y., New York State Department of Environmental Conservation report, 103 p., accessed July 19, 2023, at https://www.dec.ny.gov/docs/water_pdf/2018flwqreport.pdf.
New York State Department of Environmental Conservation [NYSDEC], 2020, Harmful algal blooms by county 2012–2019: New York State Department of Environmental Conservation report, 25 p., accessed February 1, 2023, at https://www.dec.ny.gov/docs/water_pdf/habsextentsummary.pdf.
New York State Department of Environmental Conservation [NYSDEC], 2024, Division of Water—Monitoring data portal: New York State Department of Environmental Conservation Bureau of Water Assessment and Management website, accessed May 28, 2024, at https://nysdec.maps.arcgis.com/apps/webappviewer/index.html?id=692b72ae03f14508a0de97488e142ae1.
Nunes, G.S., Toscano, I.A., and Barceló, D., 1998, Analysis of pesticides in food and environmental samples by enzyme-linked immunosorbent assays: Trends in Analytical Chemistry, v. 17, no. 2, p. 79–87, accessed February 10, 2023, at https://doi.org/10.1016/S0165-9936(97)00116-7.
O’Neil, J.M., Davis, T.W., Burford, M.A., and Gobler, C.J., 2012, The rise of harmful cyanobacteria blooms—The potential roles of eutrophication and climate change: Harmful Algae, v. 14, p. 313–334, accessed February 8, 2023, at https://doi.org/10.1016/j.hal.2011.10.027.
Prestigiacomo, A.R., Gorney, R.M., Hyde, J.B., Davis, C., and Clinkhammer, A., 2023, Patterns and impacts of cyanobacteria in a deep, thermally stratified, oligotrophic lake: AWWA Water Science, v. 5, no. 2, article e1326, 18 p., accessed May 29, 2024, at https://doi.org/10.1002/aws2.1326.
R Core Team, 2022, R—A language and environment for statistical computing, version 4.2.2 (Innocent and Trusting): R Foundation for Statistical Computing software release, accessed February 23, 2023, at https://www.R-project.org/.
Roué, M., Darius, H.T., and Chinain, M., 2018, Solid phase adsorption toxin tracking (SPATT) technology for the monitoring of aquatic toxins—A review: Toxins, v. 10, no. 4, 28 p., accessed February 15, 2023, at https://doi.org/10.3390/toxins10040167.
Sangolkar, L.N., Maske, S.S., and Chakrabarti, T., 2006, Methods for determining microcystins (peptide hepatotoxins) and microcystin-producing cyanobacteria: Water Research, v. 40, no. 19, p. 3485–3496, accessed February 8, 2023, at https://doi.org/10.1016/j.watres.2006.08.010.
Satchwell, M., and Boyer, G., 2002, Comparison of four assays for the detection of microcystins, in Steidinger, K.A., Landsberg, J.H., Tomas, C.R., and Vargo, G.A., eds., Harmful Algae 2002—Proceedings of the Xth International Conference on Harmful Algae, St. Pete Beach, Florida, USA, October 21–25, 2002: St. Petersburg, Fla., Florida Fish and Wildlife Conservation Commission, Florida Institute of Oceanography, and Intergovernmental Oceanographic Commission of UNESCO, p. 213–215. [Also available at https://myfwc.com/media/13109/xhab_detection_analytical_techniques.pdf.]
Silva, C.P., Lima, D.L., Schneider, R.J., Otero, M., and Esteves, V.I., 2014, Evaluation of the anthropogenic input of caffeine in surface waters of the north and center of Portugal by ELISA: Science of the Total Environment, v. 479–480, p. 227–232, accessed February 9, 2023, at https://doi.org/10.1016/j.scitotenv.2014.01.120.
Smith, Z.J., and Boyer, G.L., 2024, Unusual paralytic shellfish poisoning toxins in Cayuga Lake, New York: Toxicon, v. 252, article 108165, accessed January 15, 2025, at https://doi.org/https://doi.org/10.1016/j.toxicon.2024.108165.
Smith, Z.J., Conroe, D.E., Schulz, K.L., and Boyer, G.L., 2020, Limnological differences in a two-basin lake help to explain the occurrence of anatoxin-a, paralytic shellfish poisoning toxins, and microcystins: Toxins, v. 12, no. 9, article 559, accessed May 9, 2024, at https://doi.org/10.3390/toxins12090559.
Smith, Z.J., Martin, R.M., Wei, B., Wilhelm, S.W., and Boyer, G.L., 2019, Spatial and temporal variation in paralytic shellfish toxin production by benthic Microseira (Lyngbya) wollei in a freshwater New York lake: Toxins, v. 11, no. 1, 18 p., accessed March 1, 2023, at https://doi.org/10.3390/toxins11010044.
Stouder, M.D.W., Boyer, G.L., Carpenter, K.D., D’Angelo, E.M., Gorney, R.M., and Rosen, J.J., 2024, Cyanotoxin concentrations in extracts from solid phase adsorption toxin tracking (SPATT) and diffusive gradients in thin-films (DGT) samplers in Owasco Lake, Seneca Lake, and Skaneateles Lake, Finger Lakes Region, New York, 2019: U.S. Geological Survey data release, accessed January 13, 2025, at https://doi.org/10.5066/P9OZR89E.
Taranu, Z.E., Gregory‐Eaves, I., Leavitt, P.R., Bunting, L., Buchaca, T., Catalan, J., Domaizon, I., Guilizzoni, P., Lami, A., McGowan, S., Moorhouse, H., Morabito, G., Pick, F.R., Stevenson, M.A., Thompson, P.L., and Vinebrooke, R.D., 2015, Acceleration of cyanobacterial dominance in north temperate‐subarctic lakes during the Anthropocene: Ecology Letters, v. 18, no. 4, p. 375–384, accessed February 27, 2023, at https://doi.org/10.1111/ele.12420.
Trevino-Garrison, I., DeMent, J., Ahmed, F.S., Haines-Lieber, P., Langer, T., Ménager, H., Neff, J., and Merwe, D., 2015, Human illnesses and animal deaths associated with freshwater harmful algal blooms—Kansas: Toxins, v. 7, no. 2, p. 353–366, accessed February 27, 2023, at https://doi.org/10.3390/toxins7020353.
U.S. Environmental Protection Agency [EPA], 2015, Method 545—Determination of cylindrospermopsin and anatoxin-a in drinking water by liquid chromatography electrospray ionization tandem mass spectrometry (LC/ESI–MS/MS): U.S. Environmental Protection Agency report EPA 815–R–15–00, 34 p., accessed July 19, 2023, at https://www.epa.gov/sites/default/files/2017-10/documents/epa_815-r-15-009_method_545.pdf.
U.S. Environmental Protection Agency [EPA], 2016. Method 546—Determination of total microcystins and nodularins in drinking water and ambient water by adda enzyme-linked immunosorbent assay: U.S. Environmental Protection Agency report EPA 815–B–16–011, accessed July 19, 2023, at https://www.epa.gov/sites/default/files/2016-09/documents/method-546-determination-total-microcystins-nodularins-drinking-water-ambient-water-adda-enz yme-linked-immunosorbent-assay.pdf.
U.S. Geological Survey [USGS], [variously dated], National field manual for the collection of water-quality data: U.S. Geological Survey Techniques of Water-Resources Investigations, book 9, chaps. A1–A10, accessed July 19, 2023, at https://water.usgs.gov/owq/FieldManual/.
U.S. Geological Survey [USGS], 2016, USGS water data for the Nation: U.S. Geological Survey National Water Information System database, accessed March 22, 2024, at https://doi.org/10.5066/F7P55KJN.
Wiese, M., D’Agostino, P.M., Mihali, T.K., Moffitt, M.C., and Neilan, B.A., 2010, Neurotoxic alkaloids—Saxitoxin and its analogs: Marine Drugs, v. 8, no. 7, p. 2185–2211, accessed May 29, 2024, at https://doi.org/10.3390/md8072185.
Wood, S.A., Holland, P.T., and MacKenzie, L., 2011, Development of solid phase adsorption toxin tracking (SPATT) for monitoring anatoxin-a and homoanatoxin-a in river water: Chemosphere, v. 82, no. 6, p. 888–894, accessed February 8, 2023, at https://doi.org/10.1016/j.chemosphere.2010.10.055.
Zendong, Z., Bertrand, S., Herrenknecht, C., Abadie, E., Jauzein, C., Lemée, R., Gouriou, J., Amzil, Z., and Hess, P., 2016, Passive sampling and high resolution mass spectrometry for chemical profiling of French coastal areas with a focus on marine biotoxins: Environmental Science & Technology, v. 50, no. 16, p. 8522–8529, accessed May 30, 2024, at https://doi.org/10.1021/acs.est.6b02081.
Conversion Factors
International System of Units to U.S. customary units
Supplemental Information
Concentration of microcystin in water is given in micrograms per liter (µg/L).
Concentration of total phosphorus in water is given in milligrams per liter (mg/L).
Abbreviations
ADDA
(4E,6E)-3-amino-9-methoxy-2,6,8-trimethyl-10-phenyldeca-4,6-dienoic acid
cyanoHAB
cyanobacterial harmful algal bloom
ELISA
enzyme-linked immunosorbent assay
EPA
U.S. Environmental Protection Agency
LC–MS
liquid chromatography with mass spectrometry
LC–MS/MS
liquid chromatography with tandem mass spectrometry
MDL
method detection limit
NWIS
National Water Information System
NYSDEC
New York State Department of Environmental Conservation
r
Pearson’s correlation coefficient
SPATT
solid phase adsorption toxin tracking
USGS
U.S. Geological Survey
For more information about this report, contact:
Director, New York Water Science Center
U.S. Geological Survey
425 Jordan Road
Troy, NY 12180–8349
dc_ny@usgs.gov
or visit our website at
Disclaimers
Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Although this information product, for the most part, is in the public domain, it also may contain copyrighted materials as noted in the text. Permission to reproduce copyrighted items must be secured from the copyright owner.
Suggested Citation
Johnston, B.D., Stouder, M.D.W., Gorney, R.M., Rosen, J.J., Carpenter, K.D., Wei, B., and Boyer, G.L., 2025, Evaluation of passive samplers for cyanotoxin detection by immunoassay and chromatographic-mass spectrometry: U.S. Geological Survey Scientific Investigations Report 2025–5046, 37 p., https://doi.org/10.3133/sir20255046.
ISSN: 2328-0328 (online)
Study Area
| Publication type | Report |
|---|---|
| Publication Subtype | USGS Numbered Series |
| Title | Evaluation of passive samplers for cyanotoxin detection by immunoassay and chromatographic-mass spectrometry |
| Series title | Scientific Investigations Report |
| Series number | 2025-5046 |
| DOI | 10.3133/sir20255046 |
| Publication Date | June 02, 2025 |
| Year Published | 2025 |
| Language | English |
| Publisher | U.S. Geological Survey |
| Publisher location | Reston, VA |
| Contributing office(s) | New York Water Science Center, Oregon Water Science Center, Caribbean-Florida Water Science Center |
| Description | Report: vii, 37 p.; Data Release; Dataset |
| Country | United States |
| State | New York |
| Other Geospatial | Finger Lakes region |
| Online Only (Y/N) | Y |
| Additional Online Files (Y/N) | N |


