Atlantic Salmon (Salmo salar) Culture Manual
Links
- Document: Report (2.3 MB pdf) , HTML , XML
- Download citation as: RIS | Dublin Core
Acknowledgments
We would like to express our appreciation and gratitude to everyone who has participated in the Atlantic Salmon Research Program at the U.S. Geological Survey Tunison Laboratory of Aquatic Science. Without the support, guidance, and dedication of our staff members and collaborating agencies, the progress in Salmonid fish culture would not be possible. Special thanks and gratitude go to (1) all the staff at the Tunison Laboratory of Aquatic Science, specifically Jim Johnson, H. George Ketola, and Gregg Mackey; (2) staff at the New York State Department of Environmental Conservation (NYSDEC) Cape Vincent Fisheries Station, specifically Steve LaPan, Mike Connerton, and Chris Legard; (3) staff at the NYSDEC Region 7 Office (Cortland, New York), specifically Dave Lemon and Scott Prindle; (4) staff at the NYSDEC Salmon River Fish Hatchery, specifically Fran Verdoliva, Tom Kielbinski, and Andy Greulich; (5) staff at the NYSDEC Adirondack Fish Hatchery, specifically Matt Jackson; (6) staff at the Vermont Fish and Wildlife Ed Weed Fish Hatchery, specifically Kevin Kelsey; and (7) staff at the U.S. Fish and Wildlife Service Northeast Fish Health Center (Lamar, Pennsylvania) and the NYSDEC Region 6 Office (Rome, N.Y.) who graciously do the disease certification, specifically John Coll, Andrew Noyes, and Geoff Eckerland. Major funding was provided by the Great Lakes Restoration Initiative.
Abstract
The primary objective of the Atlantic Salmon Research Program established at the U.S. Geological Survey Tunison Laboratory of Aquatic Science as mandated by the Great Lakes Restoration Initiative is to restore Atlantic salmon (Linnaeus, 1758; Salmo salar) into Lake Ontario. This objective focuses on evaluating the survival of stocked Atlantic salmon in current Lake Ontario conditions to create a genetic strain that is robust to overcome current physiological and physical barriers. To complete this goal, new and innovative hatchery techniques based on past successful methods and protocols to grow Atlantic salmon to various life stages are described in this standard operating manual.
Introduction
The process of rearing Atlantic salmon (Linnaeus, 1758; Salmo salar) for conservation and restoration in their native environment, such as Lake Ontario, is a multiagency collaboration that includes adult brood collection, spawning, disease certification, genetic testing, egg incubation and development, larval and juvenile care and feeding, batch marking, and release into targeted habitats. This standard operating manual addresses all of these steps but does not deal with post release assessment to determine the efficacy of releases for restoration of conservation purposes.
Adult Brood Collection
Adult Atlantic salmon are captured from the Salmon River (not shown) between early July and the end of September as they return from Lake Ontario (not shown) to their release site (in preparation for spawning) near the New York State Department of Environmental Conservation (NYSDEC) Salmon River Fish Hatchery (lat 43.509393 °N., long 75.994579 °W.) in Altmar, New York (not shown). The fish were derived from the Sebago Lake (not shown) strain of Atlantic salmon, which is the native strain for Lake Ontario that has become extirpated from the lake. Once adult Atlantic salmon reach the hatchery, they encounter a fish ladder that typically delivers cold water to the river and is used by the U.S. Geological Survey Tunison Laboratory of Aquatic Science (TLAS) and NYSDEC personnel as a corral (fig. 1). Beach seines (2 meters [m] x 3 m; 0.08-m delta mesh) are dragged upstream slowly, forcing adult Atlantic salmon to the top of the fish ladder.

Collection of adult Atlantic salmon (Linnaeus, 1758; Salmo salar) up the Salmon River Fish Hatchery fish ladder. At the time the photograph was taken, a U.S. Geological Survey (USGS) Job Hazard Analysis was in effect during the field work. USGS scientists followed all appropriate safety protocols and requirements for working near, on, in, or over water consistent with the approved USGS Job Hazard Analysis.
Adult Atlantic salmon are then scooped out of the water with rubber fish nets and placed in an insulated transport tank (1,136 liters [L]) on a pickup truck used to transfer the fish to TLAS (fig. 2). Granulated salt (2.0 milligrams per liter [mg/L]) and liquid oxygen are supplemented during transport to reduce stress and maintain optimal conditions. Thiamine mononitrate (2,500 mg/L) is used because adults are historically thiamine deficient because of their diet while living in Lake Ontario (Ketola and others, 2000). Proper levels of thiamine are necessary for reproductive success and proper embryo and early life stage development (Ketola and others, 2000). Fish are sexed and placed in separate concrete raceways (7.3-m length, 1.5-m height, 1.5-m width) where they are held until they are ready to spawn in early to mid-November (fig. 3). The raceway is covered to provide dark conditions and supplied with a continuous flow of fresh, cold well water.

Transport tank used to transfer fish from the wild back to the U.S. Geological Survey Tunison Laboratory of Aquatic Science for spawning.

Adult Atlantic salmon (Linnaeus, 1758; Salmo salar) collected from the wild.
In late October and early November, adult Atlantic salmon are often captured from the Cayuga Inlet downstream from the NYSDEC water control structure (lat 42.427443 °N., long 76.521872 °W.; fig. 4) in Ithaca, N.Y. (not shown). With the assistance of the U.S. Fish and Wildlife Service Ecological Services electrofishing boat and crew, adult Atlantic salmon are gently stunned with electricity, scooped out of the water with rubber fish nets, and placed in a holding tank aboard the boat. Fish are transported to shore and transferred to the insulated transport tank (1,136 L) on the pickup truck for transport to TLAS. The same salt and thiamine treatments and oxygen supplementation used for Salmon River fish are applied to reduce stress and maintain optimal conditions. Fish are sexed and placed in separate concrete raceways (7.3-m length, 1.5-m height, 1.5-m width; not occupied by the Salmon River fish) and held under the same conditions as those for the Salmon River fish until they are ready to spawn in late November.

Adult Atlantic salmon (Linnaeus, 1758; Salmo salar) collected from the Cayuga Inlet, Ithaca, New York.
The demand for cultured Atlantic salmon for the array of restoration experiments usually exceeds what can be produced by wild fish returning to the Salmon River and Cayuga Inlet. Thus, Atlantic salmon eggs from NYSDEC Adirondack Fish Hatchery brood stock (Sebago Strain) in Saranac, N.Y. (not shown), are usually acquired and transferred to TLAS in late December at the eyed-up stage when they are safe to transport. Two members of the TLAS fish culture staff spend several days at the Adirondack Fish Hatchery assisting with their spawning event (fig. 5).

Spawning set up on Saranac Lake, Saranac, New York, to hold captured adult Atlantic salmon (Linnaeus, 1758; Salmo salar).
In years when local Atlantic salmon egg sources are low, additional eggs are acquired from the Vermont Fish and Wildlife Department’s Ed Weed Fish Culture Station, which collects and spawns Atlantic salmon from Lake Champlain (not shown). After the eggs have been fertilized, they are incubated on station until the eye-up stage; then, they are loaded in insulated coolers on trays wrapped in wet cheese cloth to separate them from the ice and maintain cold temperature without freezing. The eggs are then shipped to TLAS.
Spawning
Atlantic salmon spawn in late November or early December at TLAS. Ripe fish are scooped out of the raceway and immersed in a 40-L tank with raceway water and a few drops of clove oil to sedate the fish and reduce handling stress (fig. 6). In about 3 minutes, fish begin to lose swimming orientation and are ready to be spawned (fig. 7).

Sedative used to anesthetize adult Atlantic salmon (Linnaeus, 1758; Salmo salar) before they are spawned.

Holding tank used to hold adult Atlantic salmon (Linnaeus, 1758; Salmo salar) while they are being anesthetized.
The total length (in millimeters), weight (in grams), and a fin clip for genetic analysis are collected from each fish before spawning (fig. 8). Each fish is also given either an external (Floy adipose or pelvic clip) or an internal (Passive Integrated Tag [PIT] or coded wire tag) tag to identify the fish if it is recaptured after release back into the Salmon River of Cayuga Lake (not shown).

Collection of biotic data from adult Atlantic salmon (Linnaeus, 1758; Salmo salar) before they are spawned.
Spawning Atlantic salmon is outlined in this multistep process:
-
1. Dry each female Atlantic salmon with a towel to remove water contact with the eggs when they are stripped. Accidental water contact can cause the eggs to prematurely harden up before being fertilized.
-
2. Hand strip the eggs of one female Atlantic salmon into a dry bowl by applying gentle pressure to the abdominal area and sliding your hand toward the vent (fig. 9). Eggs should flow freely from the vent into the stainless-steel bowl. If the eggs are clumpy, bloody, or discolored, dispose of them and do not use them.
-
3. To maximize the genetic contribution of the population and fertilization success, fertilize the eggs from each female with the milt of two randomly selected males.
-
4. Dry each male Atlantic salmon with a towel to remove water contact with the milt when the males are stripped. Accidental water contact can cause the milt to activate prematurely before contact with the egg.
-
5. Hand strip the milt of two male Atlantic salmons into an egg-filled dry bowl by applying gentle pressure to the abdominal area and sliding your hand toward the vent (fig. 10). Milt should flow freely from the vent into the stainless-steel bowl. If blood is observed, stop immediately and use another fish.
-
6. Add 1 L of culture water to eggs and milt to activate fertilization for 1 minute and gently stir (using a soft bird feather) for 1–2 minutes to ensure all eggs are in contact with activated milt (fig. 11).
-
7. Drain and rinse the fertilized eggs three times with culture water to remove milt and any foreign particles (fig. 12).
-
8. Transfer the fertilized eggs to culture water containing 5,000 mg/L of thiamine for 45 minutes while occasionally stirring (fig. 13).
-
9. Transfer the fertilized eggs to culture water containing 10 milliliters of iodine per 1 L of water (polymeric iodine [also known as Argentyne; Argent Chemical Laboratories Inc., Redmond, Washington]) to disinfect for 10 minutes before the eggs become hardened (fig. 14). This concentration permits the treatment of about 2,000–2,500 eggs per liter.
-
10. After eggs are disinfected, they are rinsed three times with culture water to remove unabsorbed Argentyne solution.
-
11. Before the eggs are transferred to incubation trays, the total number of eggs is calculated using the Von Bayer trough (fig. 15). The eggs are lined up in the 30.5-centimeter (cm) trough and counted. For each lot of eggs, three counts are taken and averaged. This number is then looked up on a chart that indicates the number of eggs per milliliter for that lot of eggs (Troutlodge, 2019). Each lot is directly associated with a unique set of parents, and lots of eggs or fish are never mixed. This allows parents of future returning fish to be determined and also protects other lots from the need for destruction because of disease or other egg failure.

Expressing eggs from a mature female Atlantic salmon (Linnaeus, 1758; Salmo salar).

Expressing milt from a mature male Atlantic salmon (Linnaeus, 1758; Salmo salar).

Activation of milt using culture water.

Draining off excess water after eggs have been fertilized.

Thiamine immersion bath for recently spawned eggs.

Transferring eggs into an iodine solution for disinfection.

Enumeration of fertilized eggs using a Von Bayer trough.

Fertilized eggs placed in an incubation tray.
Disease Certification
Currently, the NYSDEC Fish Disease Control Unit or the U.S. Fish and Wildlife Service Northeast Fishery Center Lamar Fish Health Unit in Lamar, Pennsylvania (not shown), annually inspects all lots of Atlantic salmon for a suite of diseases, both domestic and from wild sources. The testing is in accordance with State fish health mandates requiring that fish stocked into the waters of New York are to be free of 10 harmful fish pathogens, including viral, bacterial, and parasitic forms. A subsample of all adults spawned on station is determined by the agency inspector so that the maximum number of adults can be returned alive to the system from which they were captured. That subsample is lethally sampled for disease certification. Certification takes 30–40 days, and effluent water from eggs must receive ultraviolet treatment during that period. After certification comes back negative for disease, eggs may be relocated to other containers and water need not receive ultraviolet treatment. Disease certifications for adult fish are acceptable for as much as 1 year after tests are completed. All juvenile salmon produced on station are recertified again before being stocked into New York waters. When fry are large enough to be tested (June, 5 months old), 60 fish from each lot are tested for all targeted pathogens. Because certifications are valid for as much as 1 year, fish allocated for spring smolt production do not need to be recertified unless the population was required to undergo treatment for an active pathogen.
Egg Incubation
Once eggs are in incubation trays, they must remain undisturbed until they have eyed up because strong agitation may damage them and decrease survival at this developmental stage. Atlantic salmon eggs in the incubation tray stacks are located within our wet laboratory and dedicated to Atlantic salmon rearing, where they are monitored daily for water flow, temperature, and developmental health (fig. 17).

Incubation tray stacks.
Culture water for incubation and subsequent rearing water is supplied by a 3,785-L elevated head tank. The head tank is supplied by a main well capable of producing as much as 2,271 L per minute (and several supplementary sources) and remains at a constant 9.8 degrees Celsius. The water passes through degassing columns and may be injected with oxygen from our liquid oxygen storage tank, as needed. Oxygen regulation is controlled by adjustable flow meters inside the building.
Eggs are treated with a 1:600 formalin solution for 15 minutes three times a week to control fungal growth. Once eggs reach the eye-up developmental stage, they are “shocked” by being pouring at waist height from one bucket to another with water in it, which causes dead eggs to turn a cloudy white color and makes them easier to see and remove. Dead and fungus-covered eggs are removed, and only live healthy eggs are retained and continue to develop. After the eye-up stage, eggs within each tray are picked every other day to remove dead eggs and record survival numbers. Starting 1 week before and continuing after hatching, the formalin treatment solution is reduced to 1:6,000 and applied for 1 hour. Once eggs have hatched, sac fry remain in incubation trays for about 30 days until most of their yolk sac is absorbed. Trays are still picked every other day to remove mortalities and improperly developed fry. During this 30-day period, all fry receive a thiamine mononitrate treatment (explained in the next section).
Development
After the fertilized eggs are placed in incubation trays, about 5 milliliters of eggs are viewed under a dissecting microscope attached to a microscope camera to monitor and document egg development (fig. 18).
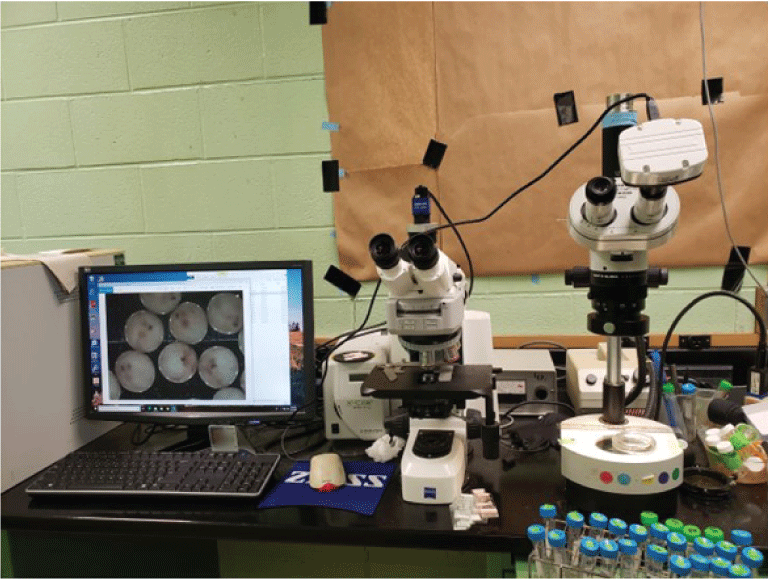
Digital dissecting microscope used to determine egg development stage.
Atlantic salmon developmental milestone stages have been identified and defined by Tunison staff and are noted as follows:
-
1. Fertilization—Recently spawned egg with the presence of lipid droplets in the egg that are spread throughout the egg and may migrate to the center of the egg (fig. 19).
-
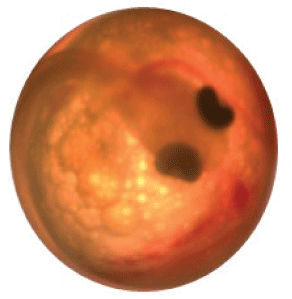
2. Eye up—The eye sockets are black with a more developed body wrapped around the chorion than in the fertilization stage (fig. 20).
-
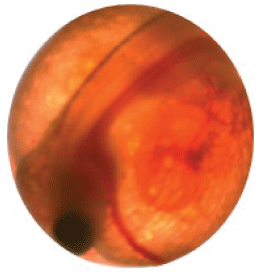
3. Heart and gill formation—The embryo is more developed and now looks like a fish larva. Gill rakers are visible with the heart beating and capillaries with blood moving (fig. 21).
-
4. Pigmentation—The embryo has a “peppering” appearance with black pigment visible all over the body (fig. 22).
-
5. Hatching—Yolk-sac larvae have clearly emerged from the egg (fig. 23).

Fertilized egg.
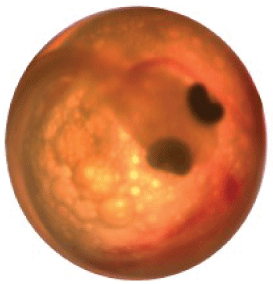
Eyed-up egg.
Egg with embryo showing a noticeable heart beating and gills moving.
Egg with embryo showing pigment on the body.
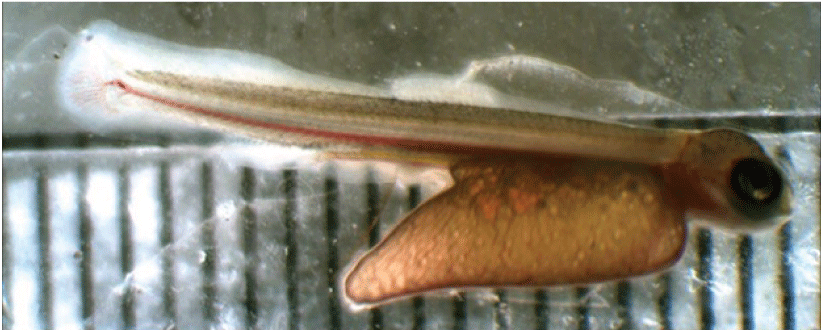
Newly hatched Atlantic salmon (Linnaeus, 1758; Salmo salar) larvae.
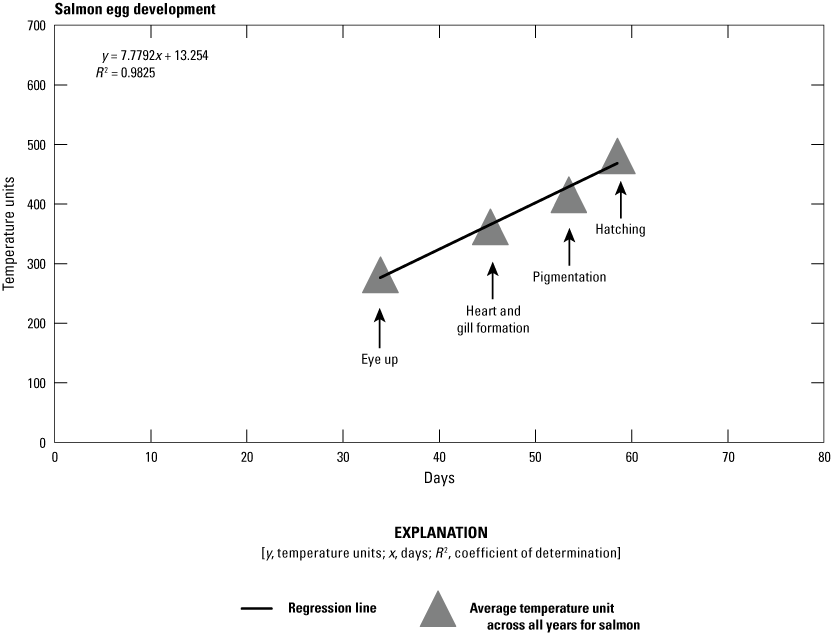
Relation among daily temperature units throughout egg development.
Larval Rearing
Atlantic salmon fry hatch in the incubation trays where they stay for the first month while absorbing nutrition from their yolk sac. During this time, all fry are treated with a thiamine mononitrate immersion to reverse physiological deficiencies that may be caused by early mortality syndrome, a lethal condition caused by thiamine deficiency within the yolk sac primarily caused by poor quality forage for the parents. Each tray is immersed in a static bath of culture water mixed with 5,000 mg/L of thiamine mononitrate (fig. 25). Because this is a static bath, additional oxygen is supplemented with electric air bubblers with air stones for 1 hour.

Equipment set up (left) for fry thiamine immersion bath (right).
Near the start of exogenous feeding, sac fry are transferred to 500-L (1.83-m diameter), conical bottom circular tanks (fig. 26). These tanks are outfitted with a central standpipe with an outer 20.3-cm collar with small holes that retain larvae but remove feces and excess food. The standpipe water level is adjusted to a depth of about 15 cm. Water comes in at surface level with slight circulation to allow fry to orient themselves and to help remove waste. Too much circular flow will force fry to the center collar and cause high mortality. A continuous supply of culture water enters the tank near the tank wall. Water inflow to each tank should allow for two to three turnovers of total volume per hour and is increased shortly after fry have reached the swim-up stage, where they are actively rising to the surface for food and swim suspended in the water column. Tanks are covered about 80 percent with black fabric to reduce stress caused by excessive light. The circular tanks are carefully cleaned daily by slightly lifting the standpipe while brushing the collected waste around the collar holes to allow flow to pull waste through the collar holes while preventing larvae from being lost to the drain. The inside and outside of the holes are brushed to remove clogging debris.
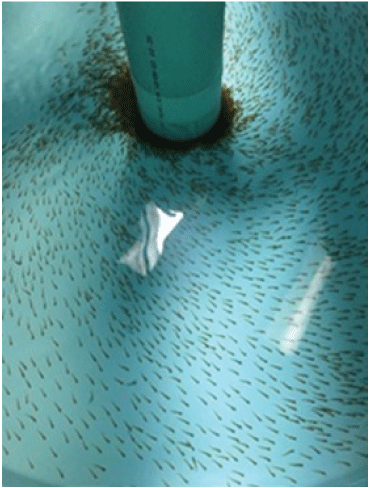
Atlantic salmon (Linnaeus, 1758; Salmo salar) fry in circular tanks.
Feeding
To maximize growth, Atlantic salmon at TLAS are continuously fed using a combination of automatic 24-hour clock feeders and daily hand feeding (fig. 27). Daily feed is calculated using a percentage of the total fish weight in grams for each tank or raceway section every 2 weeks to ensure adequate feed is supplied (table 2). To determine total fish weight, follow these steps:
-
1. Using a net, scoop a sample size of 50–75 fish (fewer than 50 is not a large enough sample, especially with smaller fish, because of excess water weight).
-
2. Determine the mass of the fish sample (in grams).
-
3. Count the number of fish in the weighed sample.
-
4. Repeat the sampling and measuring three times and calculate the mean.
-
5. Use that mean to estimate the total mass in the tank (a similar process is used for fish in raceways) based on the total number of fish in the tank.

Automatic feeders hooked on the side of the hatchery tanks.
Table 2.
Atlantic salmon (Linnaeus, 1758; Salmo salar) monthly feeding chart.[mm, millimeter]
Marking
Atlantic salmon are mechanically marked with a visible clip to differentiate hatchery-raised fish from wild fish, and the clip is clearly visible to fishermen who harvest the fish or agency personnel who monitor the fishery. Atlantic salmon at TLAS are marked in late summer with predetermined fin clip combinations associated with fish source, stocking location, and fish age at the time of stocking (fig. 28).
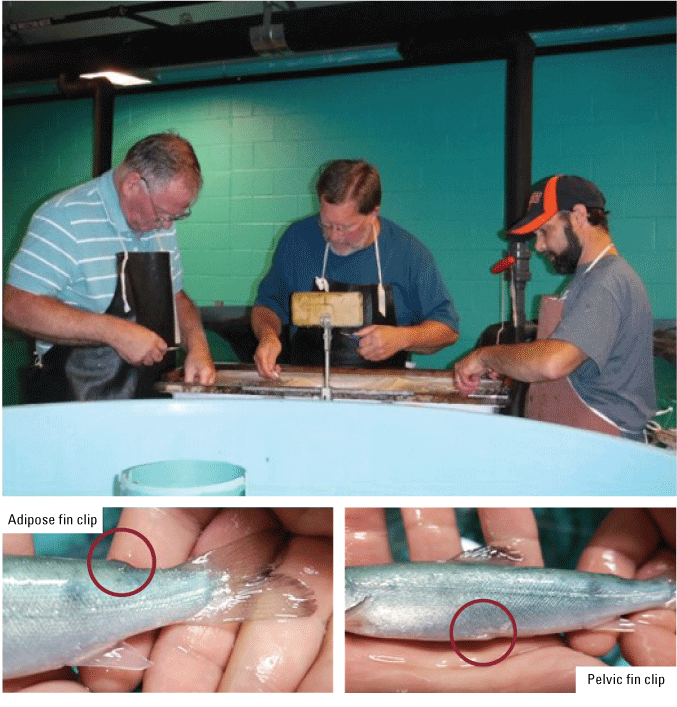
Adipose and pelvic fin clipping.
Manual Adipose Fin and Pelvic Fin Clips
The detailed steps for mechanical Atlantic salmon marking are as follows:
-
1. Remove fish from normal feeding regimen 1 day before clipping.
-
2. Set up three tubs on carts in a row so multiple people can work from the same location. The middle tub has fresh culture water and the outer tubs have culture water with 125 mg/L of tricaine methane sulfonate to anesthetize fish for safer handling.
-
3. Use a rectangular frame with netting over the top of the three tubs to allow fish to be submerged, which makes them easier to handle.
-
4. Scoop the fish and place them in a tub with netting over it and add the tricaine methane sulfonate. The fish will start to lose their balance and become sedated in 1 minute.
-
5. Once the fish are sedated, hold each fish and, with a pair of curved dissecting scissors, remove the selected fin (adipose or pelvic).
-
6. Count the clipped fish and return them to a culture tank to recover.
-
7. Fish may initially swim erratically but will return to normal behavior within minutes.
-
8. Continue to monitor the tanks and record mortality daily until the fish are released into their target habitats.
Automated Tagging and Fin Clipping
Adipose fin clipping and coded wire tag implantation can be done automatically with tagging machines. The NYSDEC has a tagging trailer that has been successfully used by personnel who clip and tag Atlantic salmon at TLAS (fig. 29). Because of the logistics of running the marking trailer, it is only used when a substantial amount of time can be saved on marking large batches of fish. The procedure is generally as follows:
-
1. Remove the fish from normal feeding regimen 1 day before clipping.
-
2. Transfer the fish from the hatchery tank to the holding tank on the tagging trailer.
-
3. The trailer system automatically transfers fish through a fish size sorter, dividing fish into separate marking sections for different size ranges.
-
4. In each section, fish move through a channel where they are briefly clamped in place, photographed, adipose fin clipped, implanted in the nose with a coded wire tag, and then photographed again to confirm clips.
-
5. Fish are then gravity fed through a quality control tube to verify the presence of a coded wire tag.
-
6. Fish passing all quality control checks are fed back inside the fish culture building to a designated tank.
-
7. Fish failing a check are collected at each station, marked by hand by technicians in the rear of the trailer, and then gravity fed to the designated tank.

New York State Department of Environmental Conservation tagging trailer.
Mark Verification
Quality control checks take place any time between 3 weeks postmarking and 1 week before stocking, which allows for tissues to heal before rehandling and ensures coded wire tags were properly placed and will not be expelled. The procedure for all tanks and raceways is as follows:
-
1. Net a random subsample of fish into a bucket with 125 mg/L of tricaine methane sulfonate.
-
2. Once the fish start to lose their balance and are sedated, they can be handled safely. Check about 50 fish to verify the fin clip and quality of the clip and examine the fish for a coded wire tag with a tag detector (Northwest Marine Technologies).
-
3. Quickly return the fish to their rearing tank to avoid unnecessary stress.
-
4. Calculate and record the percentage of verified marks for each tank.
-
5. Calculate the mean of all tanks to determine a mean for the entire lot.
Smolt Verification
Atlantic salmon go through a major physiological change called smolting before leaving their natal streams. Cultured Atlantic salmon at TLAS are inspected in the laboratory to determine the percentage of fish that show smolting morphological features (fig. 30).

Variations in the smoltification of Atlantic salmon (Linnaeus, 1758; Salmo salar). [1 denotes parr—evenly spaced dark round patches along the side of the body, 2 denotes in between parr and smolt stages, and 3 denotes smolt—disappearance of dark round patches and a silver coloration of the body]
The procedure is as follows:
-
1. For tanks with 500–1,000 fish, check a subsample of 50 randomly selected fish for smolt characteristics.
-
2. Anesthetize the fish with 125 mg/L of tricaine methane sulfonate.
-
3. Record the total length of the fish in millimeters and the weight in grams.
-
4. Check the fin margins for any black (smolt) or not black (parr) marking. Check the body for olive blue coloring (parr) or a silver shading (smolt). These are identifiable characteristics of a fish that has smolted or is smolting.
-
5. Record fish as 1 (denoting parr), 2 (denoting in between), or 3 (denoting smolt), and then return the fish to a culture tank.
Stocking
Atlantic salmon raised at TLAS are held on station for as many as 16 months and either stocked as a fall fingerling or a spring yearling. A U.S. Fish and Wildlife Service hatchery truck equipped with four 1,136-L tanks is used to transport fish to the stocking location (fig. 31).

U.S. Fish and Wildlife Service hatchery stocking truck used to stock Atlantic salmon (Linnaeus, 1758; Salmo salar) in the Salmon River.
The average weight of fish is used to determine proper stocking mass for the transport vehicle. The detailed steps in preparation of stocking are as follows:
-
1. Weigh the sample fish 1 week before the stocking event. For each raceway/tank of fish, complete the following steps:
-
2. Upon notification of the scheduled stocking day, make sure the fish are not fed 1 day before stocking so they are able to expel digested contents in the gut, which will reduce transport stress.
-
3. On the day of stocking, fill the hatchery truck tanks with culture water.
-
4. Monitor and record the water temperature and dissolved oxygen concentration (percentage and milligrams per liter, respectively).
-
5. Turn on the oxygen cylinder to add additional oxygen to water to achieve a supersaturation of about 110 percent.
-
6. Add 5,443 grams of noniodized salt to help reduce fish transport stress.
-
7. Attach a displacement gauge to determine water height before adding fish. Adjust the water level height to 25.4 millimeters for water displacement caused by the addition of fish.
-
8. Standard hatchery practice is to fill each tank on the hatchery truck at a density of 20–30 grams of fish per liter, which will raise the tank volume to 25.4 millimeters.
-
9. Once the hatchery truck is full of fish, immediately start driving to the stocking location (fig. 32).
-
a. Fingerling Atlantic salmon are stocked in Hopkinton Brook (Hopkinton, N.Y.; not shown) from shore.
-
b. Yearling Atlantic salmon smolts are stocked in the Salmon River (Salmon River Fish Hatchery, Altmar, N.Y. [not shown]; Lighthouse Marina, Port Ontario, N.Y. [not shown]) with the use of the NYSDEC stocking pontoon boat.
-

Atlantic salmon (Linnaeus, 1758; Salmo salar) being released into Hopkinton Brook or the Salmon River. At the time the photograph was taken, a U.S. Geological Survey (USGS) Job Hazard Analysis was in effect during the field work. USGS scientists followed all appropriate safety protocols and requirements for working near, on, in, or over water consistent with the approved USGS Job Hazard Analysis.
Record Keeping, Data Management, and Reporting
The proper documentation of daily fish culture practices, observation, and measurements is a critical step that is necessary during all fish culture duties. At TLAS, all documentation is written down in “Rite In The Rain” weatherproof notebooks that detail all daily fish culture processes. This information is immediately transcribed into a computer database, which ensures no loss of data and allows us to review data from previous years at any time. Reports that summarize the collection of adult fish from the wild, spawned eggs or eggs received, survival of larvae/fry/fingerlings on station, and the stocking of fish are written and submitted to the NYSDEC annually.
References Cited
Ketola, H.G., Bowser, P.R., Wooster, G.A., Wedge, L.R., and Hurst, S.S., 2000, Effects on thiamine reproduction of Atlantic salmon and a new hypothesis for their extirpation in Lake Ontario: Transactions of the American Fisheries Society, v. 129, no. 2, p. 607–612. [Also available at https://doi.org/10.1577/1548-8659(2000)129%3C0607:EOTORO%3E2.0.CO;2.]
Troutlodge, 2019, Counting eggs: Hendrix Genetics web page, accessed January 10, 2024, at https://www.troutlodge.com/en/articles/counting-eggs/.
Conversion Factors
International System of Units to U.S. customary units
Temperature in degrees Celsius (°C) may be converted to degrees Fahrenheit (°F) as follows: °F = (1.8 × °C) + 32.
Supplemental Information
Concentrations of chemical constituents in water are given in milligrams per liter (mg/L).
For more information about this publication, contact:
Director, USGS Great Lakes Science Center
1451 Green Road
Ann Arbor, MI 48105
734–994–3331
For additional information, visit: https://www.usgs.gov/centers/great-lakes-science-center
Publishing support provided by the
Rolla Publishing Service Center
Disclaimers
Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Although this information product, for the most part, is in the public domain, it also may contain copyrighted materials as noted in the text. Permission to reproduce copyrighted items must be secured from the copyright owner.
Suggested Citation
Chalupnicki, M.A., Chiavelli, R., and McKenna, J.E., Jr., 2024, Atlantic salmon (Salmo salar) culture manual: U.S. Geological Survey Techniques and Methods, book 2, chap. A21, 17 p., https://doi.org/10.3133/tm2A21.
ISSN: 2328-7055 (online)
| Publication type | Report |
|---|---|
| Publication Subtype | USGS Numbered Series |
| Title | Atlantic salmon (Salmo salar) culture manual |
| Series title | Techniques and Methods |
| Series number | 2-A21 |
| DOI | 10.3133/tm2A21 |
| Publication Date | January 18, 2024 |
| Year Published | 2024 |
| Language | English |
| Publisher | U.S. Geological Survey |
| Publisher location | Reston, VA |
| Contributing office(s) | Great Lakes Science Center |
| Description | vii, 17 p. |
| Online Only (Y/N) | Y |
| Additional Online Files (Y/N) | N |


